Abstract
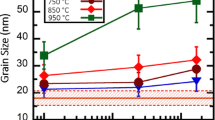
The kinetics and phase selection mechanisms involved in the crystallization of an amorphous Cu-Zr alloy of eutectic composition (Cu56Zr44) were investigated using in situ high-energy X-ray diffraction (HEXRD), transmission electron microscopy (TEM), and differential scanning calorimetry (DSC) under isothermal and constant heating rate conditions. In situ HEXRD results for 10 K/min (10 °C/min) heating indicate that the amorphous alloy devitrifies into CuZr2 and mainly Cu10Zr7 at the crystallization temperature of 725 K (452 °C). The sequence continues with the precipitation of CuZr (B2) at 1004 K (731 °C), where these three phases coexist until the decomposition of CuZr2 is observed at 1030 K (757 °C). The two equilibrium phases Cu10Zr7 and CuZr (B2) remain present on further heating until melting at the eutectic temperature for the Cu56Zr44 alloy. TEM investigation of the isothermal [705 K (432 °C)] crystallization sequence reveals primary nucleation and growth of the Cu10Zr7 phase, where growth of the Cu10Zr7 crystals is initially planar with a transition to a cellular morphology, associated with partitioning of Zr at the growth front. Related cellular structures and composition profiles are quantified.














Similar content being viewed by others
References
[1] W. Klement, R. H. Willens, P. Duwez, Nature 187 (1960) 869-870.
Y. C. Kim, J. C. Lee, P. R. Cha, J. P. Ahn, E. Fleury, Materials Science and Engineering a-Structural Materials Properties Microstructure and Processing 437 (2006) 248-253.
D.H. Xu, G. Duan, and W.L. Johnson: Phys. Rev. Lett., 2004, vol. 92.
W. Zhang, A. Inoue, Journal of Materials Research 21 (2006) 234-241.
W. Zhang, Q. S. Zhang, A. Inoue, Advanced Engineering Materials 10 (2008) 1034-1038.
W. Zhang, Q. S. Zhang, A. Inoue, Journal of Materials Research 23 (2008) 1452-1456.
Z. Altounian, G. H. Tu, J. O. Stromolsen, Journal of Applied Physics 53 (1982) 4755-4760.
R. L. Freed, J. B. Vandersande, Journal of Non-Crystalline Solids 27 (1978) 9-28.
R. L Kneller, Y. Khan, U. Gorres, Zeitschrift fur Metalkunde 77 (1986) 43-48.
[E. Kneller, Zeitschrift fur Metalkunde 77 (1986) 152-263.
C. Y. Zhang, K. F. Yao, Rare Metal Materials and Engineering 35 (2006) 158-160.
S. H. Zhou, R. E. Napolitano, Scripta Materialia 59 (2008) 1143-1146.
S. H. Zhou, R. E. Napolitano, Acta Materialia 58 (2010) 2186-2196.
S. G. Hao, C. Z. Wang, M. Z. Li, R. E. Napolitano, M. I. Mendelev, K. M. Ho, Computational Materials Science 49 (2010) 615-618.
X. W. Fang, C. Z. Wang, S. G. Hao, M. J. Kramer, Y. X. Yao, M. I. Mendelev, Z. J. Ding, R. E. Napolitano, K. M. Ho, Scientific Reports 1 (2011) 194.
I. Kalay, M. J. Kramer, R. E. Napolitano, Metall. Mater. Trans. A 42A (2011) 1144-1153.
R. L. Freed, J. B. Vandersande, Acta Metallurgica 28 (1980) 103-121.
H. R. Wang, X. Y. Teng, Z. Q. Shi, Y. F. Ye, G. H. Min, Acta Physica Sinica 50 (2001) 2192-2197.
T. E. Hosch, I. Kalay, Y. E. Kalay, M. J. Kramer, R. E. Napolitano, “Kinetics and mechanisms of isothermal devitrification in amorphous Cu50Zr50”, Metall. Mater. Trans. A 46A (2015) 600-613.
H. E. Kissinger, Journal of Research of the National Bureau of Standards 57 (1956) 217-221.
H. E. Kissinger, Analytical Chemistry 29 (1957) 1702-1706.
M. Avrami, Journal of Chemical Physics 7 (1939) 1103-1112.
M. Avrami, Journal of Chemical Physics 8 (1940) 212-224.
W. A. Johnson, R. F. Mehl, Transactions of the American Institute of Mining and Metallurgical Engineers 135 (1939) 416-442.
Acknowledgments
This work was supported by the U.S. Department of Energy (DOE), Office of Basic Energy Science, Division of Materials Science and Engineering. The research was performed at the Ames Laboratory, which is operated for the U.S. DOE by Iowa State University under contract DE-AC02-07CH11358. The high-energy X-ray experiments were performed at the MUCAT sector of the Advanced Photon Source, Argonne National Laboratory, under Grant No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 8, 2014.
Rights and permissions
About this article
Cite this article
Kalay, I., Kramer, M.J. & Napolitano, R.E. Crystallization Kinetics and Phase Transformation Mechanisms in Cu56Zr44 Glassy Alloy. Metall Mater Trans A 46, 3356–3364 (2015). https://doi.org/10.1007/s11661-015-2921-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-015-2921-5