Abstract
Summary
The National Osteoporosis Guideline Group (NOGG) has revised the UK guideline for the assessment and management of osteoporosis and the prevention of fragility fractures in postmenopausal women, and men age 50 years and older. Accredited by NICE, this guideline is relevant for all healthcare professionals involved in osteoporosis management.
Introduction
The UK National Osteoporosis Guideline Group (NOGG) first produced a guideline on the prevention and treatment of osteoporosis in 2008, with updates in 2013 and 2017. This paper presents a major update of the guideline, the scope of which is to review the assessment and management of osteoporosis and the prevention of fragility fractures in postmenopausal women, and men age 50 years and older.
Methods
Where available, systematic reviews, meta-analyses and randomised controlled trials were used to provide the evidence base. Conclusions and recommendations were systematically graded according to the strength of the available evidence.
Results
Review of the evidence and recommendations are provided for the diagnosis of osteoporosis, fracture-risk assessment and intervention thresholds, management of vertebral fractures, non-pharmacological and pharmacological treatments, including duration and monitoring of anti-resorptive therapy, glucocorticoid-induced osteoporosis, and models of care for fracture prevention. Recommendations are made for training; service leads and commissioners of healthcare; and for review criteria for audit and quality improvement.
Conclusion
The guideline, which has received accreditation from the National Institute of Health and Care Excellence (NICE), provides a comprehensive overview of the assessment and management of osteoporosis for all healthcare professionals involved in its management. This position paper has been endorsed by the International Osteoporosis Foundation and by the European Society for the Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases.
Similar content being viewed by others
Introduction
This updated guideline has been prepared, with the support of the societies listed (Appendix 1), to provide guidance on the prevention and treatment of osteoporosis with the overarching aim of reducing fragility fracture risk. This guideline updates previous National Osteoporosis Guideline Group (NOGG) guidance [1,2,3]. The scope of the guideline is to review the assessment and diagnosis of osteoporosis, the therapeutic interventions available and the approaches for the prevention of fragility fractures, in postmenopausal women, and in men aged 50 years or older. This focus is chosen as fragility fractures and osteoporosis are uncommon in premenopausal women, and men younger than 50 years and therefore when these occur patients need thorough investigation for secondary causes of osteoporosis, and careful consideration of treatment options. Specialist referral is usually required.
This NOGG guidance has appraised the current evidence-based to inform these updated recommendations. The aim of the guideline is to provide clinically appropriate recommendations which integrate available evidence on clinical efficacy, effectiveness and safety. This contrasts with, but complements, the remit of the National Institute for Health and Care Excellence (NICE), which focuses principally on establishing criteria for cost-effectiveness. Cost-effectiveness analyses are generally supportive for treatment guided by clinical effectiveness thresholds, rather than defining intervention thresholds per se [4]. The NOGG recommendations have been previously demonstrated to be cost-effective and at the time of writing, NICE’s appraisal of romosozumab is awaited, with preliminary evidence of its cost-effectiveness established [5]. The guideline has been prepared by a writing group and has been approved after consultation with stakeholders (Appendix 1).
The guideline is intended for all healthcare professionals involved in the prevention and treatment of osteoporosis and fragility fractures. This includes primary care practitioners, allied health professionals, and relevant specialists in secondary care including rheumatologists, gerontologists, gynaecologists, endocrinologists, clinical biochemists, and orthopaedic surgeons. The guideline includes recommendations for training in osteoporosis care. The conclusions and recommendations in the document are systematically graded, according to the quality of information available, to indicate the level of evidence on which recommendations are based. The grading methodology is summarised in Appendix 2. Where available, systematic reviews, meta-analyses, and randomized controlled trials have been used to provide the evidence base. The evidence base has been updated using PubMed to identify systematic reviews and meta-analyses from July 2016 to September 2020. The quality of systematic reviews and meta-analyses used in the formulation of recommendations was assessed using AMSTAR2 [6] (Appendix 3). The recommendations in this guideline were agreed upon by the National Osteoporosis Guideline Development Group.
This guideline provides a framework from which local management protocols should be developed to provide advice for healthcare professionals. Implementation of this guideline should be audited at a local and national level. The recommendations in the guideline should be used to aid management decisions but do not replace the need for clinical judgment in the care of individual patients in clinical practice.
Background
The conceptual definition of osteoporosis was made by the World Health Organization (WHO) in 1994 as a “progressive systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture” [7]. Since microarchitectural deterioration could not be measured clinically, the operational description was based on a bone mineral density (BMD) T-Score of ≤ − 2.5. Over the years, this was adopted as a clinical definition; however, the limitations of focusing on a BMD-based definition alone have since become clear. BMD is now viewed as one, albeit very important, risk factor to be considered when assessing fracture risk which is now viewed as the principal necessity.
The clinical significance of osteoporosis lies in the fractures that arise. Approximately one in two adult women and one in five men will sustain one or more fragility fractures (a low trauma fracture sustained from a fall from standing height or less) in their lifetime [8]. In the UK, the prevalence of femoral neck BMD T-Score ≤ − 2.5, in those aged 50 years and older, is 6.8% in men and 21.8% in women [9]. However, the majority of people who sustain a fragility fracture will have a femoral neck BMD T-Score above − 2.5, reflecting the contribution of many other factors, besides BMD, to fracture risk [10,11,12]. Fall-related risk factors add significantly to fracture risk and often overlap with risk factors for osteoporosis, hence the need for integrated fall and fracture services.
Currently, in the UK, approximately 549,000 new fragility fractures occur each year, including 105,000 hip fractures, 86,000 vertebral fractures, and 358,000 other fractures (i.e., fractures of the pelvis, ribs, humerus, forearm, tibia, fibula, clavicle, scapula, sternum, and other femoral fractures); 33% are sustained by men [9, 13, 14]. Such fractures cause severe pain, disability, and reduction in quality of life [15, 16]. In the UK, fragility fractures are estimated to account for 579,722 DALYs (Disability Adjusted Life Years) lost, largely driven by years lived with disability. This equates to 24 DALYs per 1000 people aged over 50 years, which is comparable to the DALYs lost from dementia [9]. Costs of fragility fractures to the National Health Service (NHS) exceed £4.7 billion per annum, of which £2.6 billion is directly incurred after an incident fracture (£1.1 billion for hip fractures alone [17]), with more than £1.7 billion attributable to institutional care costs post-fracture (estimated for 2017) [9]. Total direct costs for 2019 were £5.4 billion accounting for 2.4% of healthcare spending [18].
Common sites of fragility fracture include the vertebral bodies, hip, distal radius, proximal humerus, and pelvis. Hip fracture is the most common reason for emergency anaesthesia and surgery in older people. It is also the most common cause of death following a fall. After hip fracture the mean hospital length of stay is 20 days, accounting for half a million hospital bed days used each year, with 3,600 hospital beds (3,159 in England, 325 in Wales, and 133 in Northern Ireland) occupied at any one time by patients recovering from hip fracture [19, 20]. Loss of independence is common following a hip fracture with only 52% living in their own home after 120 days [13] and 26% will die within 12 months of their fracture [21]. Most major osteoporotic fractures are associated with reduced relative survival, part causally related and part due to associated co-morbidity [22,23,24].
In the UK, fracture rates vary by geographic location, race and levels of socioeconomic deprivation [25,26,27]. As in many higher-income countries, age- and sex-adjusted fracture rates appear relatively stable, although increases in hip fractures amongst men in the UK have been reported [25, 28]. Changes in vertebral fracture rates potentially reflect secular alterations to reporting of cases. Importantly, the ageing of the UK population is predicted to give rise to a 19.6% increase in the number of fragility fractures by 2030 if changes are not made to current practice [9].
Fracture risk assessment and case finding
Recommendations
-
1.
A FRAX assessment should be performed in any postmenopausal woman, or man age ≥ 50 years, with a clinical risk factor for fragility fracture, to guide BMD measurement and prompt timely referral and/or drug treatment, where indicated (strong recommendation).
-
2.
When using FRAX to calculate the probability of fracture, clinical judgement is needed when clinical risk exceeds those factors able to be entered into FRAX (strong recommendation).
-
3.
Arithmetic adjustments to FRAX probabilities of major osteoporotic fracture (MOF: clinical spine, hip, forearm or humerus) and hip fracture (Table 1) can be used in clinical practice, to take account of additional clinical risk factors, such as glucocorticoid use, discordantly low lumbar spine BMD, type II diabetes, and a history of falls (conditional recommendation).
-
4.
Vertebral fracture assessment (VFA) is indicated in postmenopausal women, and men age ≥ 50 years, if there is a history of ≥ 4 cm height loss, kyphosis, recent or current long-term oral glucocorticoid therapy, a BMD T-score ≤ − 2.5 at either the spine or hip, or in cases of acute onset back pain with risk factors for osteoporosis (strong recommendation).
-
5.
T-scores in men and women derived from femoral neck BMD should use normative values for BMD derived from young healthy women from NHANES III (strong recommendation).
-
6.
DXA scan results should be reported within 3 weeks of the scan, by healthcare professionals with specific training in DXA interpretation, and in accordance with national and international reporting standards (strong recommendation).
-
7.
Patients with osteoporosis and/or a fragility fracture should be investigated for underlying causes, this includes the need for routine blood tests (strong recommendation).
-
8.
The use of quantitative ultrasound is not recommended for the diagnosis of osteoporosis (strong recommendation).
-
9.
QCT-measured femoral neck areal BMD in postmenopausal women, and men age ≥ 50 years, can be used for opportunistic diagnosis of osteoporosis and to inform individual treatment decisions using FRAX (conditional recommendation).
-
10.
Computer-Aided Diagnostics (CAD) may be considered to improve standard reporting of CTs performed on postmenopausal women, and men age ≥ 50 years, to improve opportunistic identification of vertebral fractures (conditional recommendation).
Measurement of bone mineral density
The risk of fracture increases progressively with decreasing bone mineral density (BMD). Systematic reviews and meta-analyses of observational population-based studies using absorptiometric techniques indicate that the risk of fracture increases approximately two-fold for each standard deviation (SD) decrease in BMD [29, 30]; (evidence level Ia). The gradient of fracture risk varies according to the site and technique used, the person’s age and the fracture type [30]; (evidence level Ia). The predictive value of BMD for hip fracture is at least as good as that of blood pressure for stroke [31]; (evidence level IV).
The WHO and the International Osteoporosis Foundation (IOF) recommend that the reference technology for the measurement of BMD is dual-energy X-ray absorptiometry (DXA) applied to the femoral neck, because of its higher predictive value for fracture [32, 33]; (evidence level Ia). DXA measurements of femoral neck BMD are used in FRAX®. The spine is not always a reliable site for risk assessment or for the diagnosis of osteoporosis in older people because of the high prevalence of degenerative changes, which artefactually increase the BMD value. However, a result in an older person showing low BMD is almost always valid and clinically useful, particularly in those people with disproportionately low spine BMD compared to the hip. At the same DXA-measured femoral neck BMD, men and women are at approximately the same fracture risk [34, 35]; (evidence level IIa). Therefore, the recommended reference range, from which femoral neck and total hip T-scores are calculated for men, women and transgender individuals in the US, is that derived from the NHANES III survey for white women aged 20–29 years [33, 36]. The reference ranges, from which lumbar spine and distal forearm T-scores are calculated, for both men and women of all ethnicities, are usually those of the manufacturer of the DXA scanner [36].
Osteoporosis can be diagnosed on the basis of the BMD T-score measured at the total hip, femoral neck, or lumbar spine. However, fracture risk prediction is not improved by the use of measurements from multiple sites [37, 38]; (evidence level IIa). Where hip BMD measurement is not possible for technical reasons, or if the spine is differentially affected, then spine BMD measurements can be used for diagnosis. A diagnosis of osteoporosis can be made based on the distal forearm (1/3 radius) T-score if neither spine nor hip can be reliably measured or interpreted, or if a patient exceeds the weight limit for the DXA table [36]; (evidence level IV). Serial BMD measurement can be used to monitor response to treatment [39]. Lumbar spine BMD shows the largest treatment-related changes and is the preferred site, although if spinal degenerative changes are marked, BMD at the hip is a better site for monitoring. The validity of BMD measurements depends on good quality control and national (Royal Osteoporosis Society) and international (International Society for Clinical Densitometry) bodies have published standards for the reporting of DXA scans [36, 40].
QCT-measured femoral neck areal BMD predicts osteoporotic fractures in men and women and is equivalent to DXA-derived areal BMD [41,42,43]. Femoral neck and total hip T-scores calculated from two-dimensional projections of quantitative computed tomography (QCT) data are equivalent to the corresponding DXA-derived T-scores. Thus, femoral neck CT X-ray absorptiometry (CTXA) BMD measurements can be included in FRAX [36, 44,45,46]; (evidence level IIa). Other techniques for assessing skeletal BMD, including quantitative ultrasound, have been less well-validated than absorptiometric techniques.
Assessment of clinical risk factors
The performance characteristics of BMD assessment can be improved by the concurrent consideration of clinical risk factors that operate independently of BMD. Of particular importance is age, which contributes to risk independently of BMD [12, 47]; (evidence level Ia). Additional clinical risk factors have been identified that provide information on fracture risk independently of both age and BMD:
-
i.
Low body mass index (BMI) is a significant risk factor for hip fracture, but the value of BMI in predicting other fractures is very much diminished when adjusted for BMD [48]; (evidence level Ia).
-
ii.
A history of a prior fracture, particularly if sustained from low trauma and at a site characteristic for osteoporosis, is an important risk factor for further fracture [49]. The risks are in part independent of BMD [50]. Fracture risk is approximately doubled in the presence of a prior fracture, including asymptomatic moderate or severe (grade 2 or 3) morphometric vertebral fractures [50, 51]; (evidence level Ia). The increase in risk is even more marked for more than one vertebral fracture. After a fracture, the risk of subsequent fracture is highest in the immediate post-fracture interval (imminent risk) with more than one-third of subsequent fractures over a ten-year time frame occurring within the first year [52, 53]; (evidence level Ic).
-
iii.
A parental history of hip fracture is a significant risk factor that is largely independent of BMD [54]; (evidence level Ia).
-
iv.
Smoking is a risk factor that is in part dependent on BMD [55]; (evidence level Ia).
-
v.
Oral glucocorticoid therapy increases fracture risk in a dose-dependent manner. The fracture risk conferred by the use of glucocorticoids is, however, not solely dependent upon bone loss and BMD-independent risks have been identified [56, 57]; (evidence level Ia).
-
vi.
Alcohol intake shows a dose-dependent relationship with fracture risk. Where alcohol intake is on average two units or less daily, no increase in risk has been identified. Intakes of 3 or more units daily are associated with a dose-dependent increase in fracture risk [58]; (evidence level Ia).
-
vii.
There are many secondary causes of osteoporosis (e.g., inflammatory bowel disease, endocrine disorders), but in most instances, it is uncertain to what extent an increase in fracture risk is dependent on low BMD or other factors such as the use of glucocorticoids. By contrast, rheumatoid arthritis increases fracture risk independently of BMD and the use of glucocorticoids [57]; (evidence level Ia).
-
viii.
Diabetes mellitus (both type I and type II) is associated with an increase in the risk of hip and non-vertebral fracture. In type II diabetes; a longer duration of disease and insulin use is associated with an increased risk [59, 60]; (evidence level Ia), which is partly independent of BMD [61, 62].
The use of combined clinical risk factors alone to predict fracture risk performs very similarly to that of BMD alone [63]. The use of clinical risk factors with the addition of BMD is optimal, but BMD measurement can be targeted to those close to the threshold of low/high risk or close to the threshold of high/very high risk. There are many additional clinical risk factors for fracture not included in FRAX, including risks that either act solely by reducing BMD, or have been less well-validated, or identify a risk that may not be amenable to particular treatments [12, 64]. Liability to falls is an example of the latter where the risk of fracture is high, and treatment with drugs affecting bone metabolism alone may not fully address this risk [65].
In addition to glucocorticoids, several medications are known to increase hip fracture risk including thyroid hormone excess, aromatase inhibitors for the treatment of breast cancer and androgen deprivation for the treatment of prostate cancer [66,67,68,69,70]; (evidence level Ia). Thiazolidinediones, used in the treatment of type II diabetes also increase fracture risk [71, 72]. Several other drugs have been associated with increased fracture risk including antidepressants, antiparkinsonian drugs, antipsychotic drugs, anxiolytic drugs, benzodiazepines, sedatives, H2 receptor antagonists and proton pump inhibitors [66,67,68,69,70]. The extent to which fracture risk is mediated by low BMD, falls risk or other factors, or indeed is definitely causal in each case, is not known. The impact of sex steroids on bone health in transgender individuals is unclear [73]. Biochemical indices of skeletal turnover have the potential to aid risk assessment but probably play a more immediate role in the monitoring of treatment [74,75,76]; (evidence level Ia).
Fracture risk assessment tools
The IOF and the WHO recommend that risk of fracture is expressed as absolute risk, i.e., probability over a ten-year interval [12]. The absolute risk of fracture depends upon age and life expectancy as well as the current relative risk. The period of 10 years covers the likely initial duration of treatment and the benefits that may continue if treatment is stopped. Shorter time horizons do not aid the categorisation of risk [77, 78]. Algorithms that integrate the weight of clinical risk factors for fracture risk, with or without information on BMD, were developed in 2008 by the then WHO Collaborating Centre for Metabolic Bone Diseases at Sheffield. The FRAX tool (www.shef.ac.uk/FRAX) computes the 10-year probability of hip fracture and/or of major osteoporotic fracture. A major osteoporotic fracture is a clinical spine, hip, forearm or humerus fracture. The tool has been externally validated in independent cohorts [47, 79]; (evidence level Ia).
QFracture is based on a UK prospective open cohort study of routinely collected data from general practices that take into account numerous clinical risk factors and estimate the 1- to 10-year cumulative incidence of hip and/or major osteoporotic fracture (http://www.qfracture.org [80]). The NICE has recommended the use of fracture risk assessment tools (FRAX or QFracture) in the assessment of patients [81]. Since FRAX and QFracture yield different outputs (probability of fracture accounting for mortality risk in the case of FRAX, and cumulative risk of fracture in the case of QFracture), the two calculators cannot be used interchangeably. In addition, BMD cannot be incorporated into QFracture estimations. Finally, the NOGG intervention thresholds, recommended by NICE Quality Standards, are based on FRAX probability and thus cannot be used with fracture risk derived from QFracture or other calculators [79, 82].
The input into FRAX includes, with age and sex, BMD independent clinical risk factors including the following:
Body mass index (calculated from weight and height in kg/m2), previous fragility fracture (including morphometric vertebral fracture), parental history of hip fracture, current glucocorticoid treatment (any dose, by mouth for 3 months or more), current smoking, alcohol intake 3 or more units daily, rheumatoid arthritis, secondary causes of osteoporosis (including type I diabetes, long-standing untreated hyperthyroidism, untreated hypogonadism/premature menopause (< 45 years), chronic malnutrition/malabsorption, chronic liver disease, non-dialysis chronic renal failure (i.e., CKD 3a–5). Femoral neck BMD is an optional input. The listed secondary causes are conservatively assumed to be mediated through low BMD and carry no weight when femoral neck BMD is entered into FRAX.
FRAX assessment takes no account of prior osteoporosis drug treatment, or of the dose of several clinical risk factors. For example, a history of two prior fractures carries a higher risk than a single prior fracture. A prior clinical vertebral fracture carries an approximately two-fold higher risk than other prior fracture types. Dose responses are also evident for glucocorticoid use and are partially addressed in the NOGG guideline. Since it is not possible to model all such scenarios within the FRAX algorithm, clinical judgement is needed to interpret FRAX outputs.
High- and low-impact injuries exist on a continuum and the clinical significance of high and low impact fractures is blurred in the context of osteoporosis. Indeed, prior high-trauma fractures are associated with low BMD and future fracture risk to the same extent as fractures without high trauma [49]. Although FRAX has a limited input of variables, relatively simple arithmetic procedures are available (Table 1) which can be applied to conventional FRAX estimates of probabilities of hip fracture and major osteoporotic fracture to adjust the probability assessment with knowledge of high, moderate, and low exposure to oral glucocorticoids [83]; (evidence level IIa), concurrent data on lumbar spine BMD [84, 85]; (evidence level Ia), information on the trabecular bone score (TBS—values can be entered on the UK FRAX website) [86]; (evidence level Ia), hip axis length [87]; (evidence level Ib), falls history [88]; (evidence level IIa), country of birth [89]; (evidence level Ib), type II diabetes mellitus [90]; (evidence level Ib), and recent major osteoporotic fracture (MOF) [53]; (evidence level Ib). When applying these FRAX adjustments, a suggested increase of x% should be applied as a proportion of the original FRAX score. For example, uplifting the FRAX probability of 30% by 10% gives an adjusted probability of 30 × 1.10 = 33%. There is no evidence base available to inform on the accuracy of multiple adjustments. Pragmatically, the adjustment should be made for the most dominant factor, i.e. that which will have the greater impact on the estimated probability; (evidence level IV). Although type I diabetes carries a risk of fracture over and above that provided by FRAX, there are yet no empirical data from which to recommend adjustment. In the meanwhile, the same adjustment can be used for type II diabetes; (evidence level IV). Additionally, FRAX values have been shown to be largely unaffected by socioeconomic status [91], variation in body composition [92], and chronic renal disease [93]; (evidence level Ib). Adjustments to FRAX probabilities which take into account severity and/or number of vertebral fractures cannot currently be made because of the lack of appropriate empirical data.
Risk is best presented to patients numerically using simple frequencies and positive and negative framing, e.g., for a 23% risk say ‘100 people like you, over the next 10 years, 23 will break a bone and 77 will not’. Describing risks solely with words, such as ‘You have a high chance of experiencing a fracture’ is ineffective and does not provide patients with the details needed to make an informed decision; it increases risk perceptions, and patients vary in their interpretations of what are low and high risks. It is easier for patients to understand whole numbers and simple frequencies (e.g., 1 in 100) rather than percentages. Graphs and pictograms make numeric information easier to understand and should be used where available [94]; (evidence level IV).
Investigation of osteoporosis and fragility fractures
Diagnostic assessment of individuals with osteoporosis should exclude diseases that mimic osteoporosis, identify the cause(s) of the osteoporosis, and include the management of any associated comorbidity. Common investigations are given in Table 2.
Vertebral fracture assessment
The majority of vertebral fractures do not currently come to medical attention and thus remain undiagnosed [95]. Moderate or severe vertebral fractures, even when asymptomatic, are strong risk factors for subsequent fracture at the spine and other skeletal sites [51, 96, 97]; (evidence level Ia). Vertebral fracture assessment (VFA) should therefore be considered in high-risk individuals, using either lateral lumbar and thoracic spine radiographs or lateral spine DXA imaging [98]; (evidence level Ia). The latter delivers a significantly lower radiation dose whilst performing comparably to traditional radiographs [99]. Identification of vertebral fractures on routine radiological images, such as plain abdominal and chest radiographs, performed for other indications, offers the opportunity to detect clinically important osteoporotic fractures. Opportunistic diagnosis of osteoporosis and vertebral fractures is feasible using CT scans acquired for various clinical reasons since the hip and spine are frequently in the scan field [100]; (evidence level Ia). Vertebral fracture identification from CT using Computer Aided Diagnostics (CAD) can augment and improve standard reporting methods [101,102,103,104]; (evidence level IIb). Reliable CAD methods have high sensitivity, specificity, and accuracy for vertebral fracture detection; (evidence level IV).
Screening and case finding
At present, there is no universally accepted policy for population-based screening to identify people with osteoporosis. With the recognition that factors in addition to BMD can improve fracture risk prediction, it is possible that screening strategies might be implemented in the future.
A trial of screening in the UK used FRAX to target osteoporosis drug treatment to women at high risk of hip fracture. The risk assessment, with subsequent femoral neck BMD measurement and input to FRAX in intermediate-/high-risk individuals, was conducted in a primary care setting and involved almost 12,500 women aged 70–85 years. Over 5 years, compared to standard clinical care, the screening program reduced the number of hip fractures by 28%. Similar results were observed in a study from Denmark [105], but with lesser effects observed in a further study in the Netherlands [106]. A meta-analysis of the three trials showed that screening reduced hip fracture risk by 20% [107]; (evidence level Ia).
In the absence of a screening policy, a case-finding strategy is appropriate where patients are identified because of a fragility fracture or by the presence of other clinical risk factors. There are many clinical risk factors for fracture in addition to those included in FRAX which can be used to trigger fracture risk assessment (Table 3), including thoracic kyphosis and height loss (> 4 cm), either in comparison with recalled young adult height or a documented loss on serial measurements [108]; (evidence level IIa), and bariatric surgery resulting in malabsorption [109]; (evidence level Ia).
Intervention thresholds and strategy
Recommendations
-
1.
An initial FRAX assessment, which provides the ten-year probability of a major osteoporotic fracture (MOF; clinical spine, hip, forearm or humerus) and/or hip fracture, can be used to identify patients at low, intermediate, high or very high risk of fracture (strong recommendation).
-
2.
Consider, particularly in older people, drug treatment in those with a prior and/or recent fragility fracture, with fracture risk assessment informing the choice of drug treatment (strong recommendation).
-
3.
Men and women with high and very high fracture risk (see Fig. 1) should have a DXA if a baseline measurement is needed against which to compare future BMD measurements (strong recommendation).
-
4.
Men and women with intermediate fracture risk (i.e., between the upper and lower assessment thresholds) should be referred for BMD measurement, if practical. Thereafter, fracture probability should be reassessed using FRAX (strong recommendation).
-
5.
When BMD is included in a FRAX assessment, the patient’s risk (high, very high or low) is determined by the higher of the two (MOF and hip fracture) risk assessments (strong recommendation).
-
6.
In men and women with intermediate fracture risk, if BMD measurement is unavailable, contraindicated, or impractical (e.g., in frail individuals), drug treatment should be offered if there is a history of fragility fracture and/or if fracture risk exceeds the intervention threshold (strong recommendation).
-
7.
Men and women with low fracture risk, without a prior fragility fracture, can be reassured that their fracture risk is low and offered lifestyle advice as appropriate (strong recommendation).
-
8.
Consider referral of very high-risk patients to an osteoporosis specialist in secondary care, for assessment and consideration of parenteral treatment (some may need first-line anabolic drug treatment, especially those with multiple vertebral fractures). Indications for specialist referral include (conditional recommendation):
-
⚬ The presence of single but important clinical risk factors, such as the following:
-
A recent vertebral fracture (within the last 2 years)
-
≥ 2 vertebral fractures (whenever they have occurred)
-
BMD T-score ≤ − 3.5
-
Treatment with high dose glucocorticoids (≥ 7.5 mg/day of prednisolone or equivalent over 3 months) (refer urgently given rapid loss in bone post-initiation of glucocorticoids; if any delay is anticipated, start an oral bisphosphonate in the meantime)
-
⚬ The presence of multiple clinical risk factors, particularly with a recent fragility fracture indicating a high imminent risk of re-fracture
-
⚬ Or other indicators of very high fracture risk.
-
9.
The choice of drug treatment should be informed by the level of fracture risk, additional clinical risk factors, cost-effectiveness of treatment and patient preferences (strong recommendation).
-
10.
FRAX and the link to the NOGG website should be incorporated into electronic patient health record systems (strong recommendation).
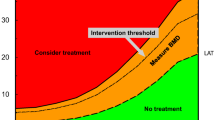
NOGG assessment, intervention, and risk thresholds for major osteoporotic fracture probability (MOF) in the UK with the use of FRAX. Individuals with probabilities below the lower assessment threshold (LAT) are considered for lifestyle advice. Those at intermediate risk (probabilities between the upper assessment threshold (UAT) and lower assessment threshold (LAT) are further assessed with BMD measurement. Where probabilities calculated using BMD lie above or below the intervention threshold (IT), treatment or lifestyle advice, respectively, is recommended [3, 79]. Patients with probabilities above the upper assessment threshold (UAT) are considered for treatment. Those with probabilities above the very high-risk threshold (VHRT) should be considered for specialist referral. Where BMD measurement is not practical (e.g., when individuals are frail and unable to get onto a DXA table, or lie flat on a DXA table), patients with probabilities above the IT are considered for treatment
FRAX assessment thresholds for 10-year probability of fracture
The approach recommended for decision-making is based on fracture probabilities derived from FRAX and can be applied to men and women [79]. This approach is underpinned by cost-effectiveness analysis with oral or intravenous bisphosphonates as the intervention [110, 111]; (evidence level Ib). FRAX assessment thresholds for 10-year probability of a major osteoporotic fracture (MOF) are shown in Fig. 1. The use of FRAX without BMD has approximately the same performance as BMD without FRAX [12]; (evidence level Ia). Thus, the same intervention threshold can be used when fracture risk is assessed with or without BMD (see Fig. 1). For men and women, the intervention threshold up to age 70 years is set at a risk equivalent to that of a woman of the same age with a prior fracture, in line with current clinical practice, and therefore rises with age. At age 70 years and above, fixed thresholds are applied [112]; (evidence level Ib). The proportion of women potentially eligible for treatment rises from approximately 30% to 50% with age, largely driven by the prevalence of prior fracture [112]; (evidence level Ib). When FRAX is calculated with BMD included, the NOGG website also provides intervention thresholds based on the 10-year probability of hip fracture, in addition to the 10-year probability of a MOF (Fig. 2). If there is discordance between the risk categories identified by the two probabilities, the highest risk category can be used to guide intervention. Of note, in the SCOOP study of screening for high fracture risk, treatment was targeted on the basis of risk assessed by hip fracture probability, with or without BMD [113].
NOGG thresholds for intervention and/or referral using major osteoporotic fracture (MOF) and hip fracture (HF) probabilities in the UK. The panels show the thresholds following the recalculation of FRAX after the input of BMD; the same thresholds are used when BMD is unavailable. The intervention threshold (IT) and very high-risk threshold (VHRT) denote the thresholds for high and very high risk, respectively
Indications for specialist referral in those at very high-fracture risk
Individuals at very high fracture risk have the most to gain from a thorough investigation of osteoporosis, falls assessment, and development and delivery of a personalised treatment plan for a chronic, life-long condition. A number of treatments now available to treat osteoporosis are mostly (but not exclusively) initiated through secondary care, and the sequence in which they are used is important; for example, the two available anabolic agents (teriparatide and romosozumab) are licensed for once only treatment courses. Treatment with teriparatide or romosozumab, which are anabolic skeletal agents, result in rapid and greater fracture risk reductions than some antiresorptive treatments [114,115,116]; (evidence level Ib). This has led to the need to identify the sub-group of patients at very high fracture risk who would potentially benefit from clinical review by an osteoporosis specialist, and who may benefit from anabolic drug treatment [117]. Indications for referral to an osteoporosis specialist may arise through several routes, for example in the presence of single but important clinical risk factors, such as a recent vertebral fracture (within the last 2 years), ≥ 2 vertebral fractures (whenever they have occurred), a BMD T-score ≤ − 3.5, high-dose glucocorticoids use (≥ 7.5 mg/day of prednisolone or equivalent over 3 months) [56, 118]; (evidence levels IIb and IV), or via a combination of clinical risk factors, resulting in very-high-fracture risk [119]; (evidence level IIb).
Prior fragility fracture is a well-established risk factor for a future fracture. This risk of subsequent osteoporotic fracture is particularly acute immediately after an index fracture and wanes progressively over the next 2 years, but thereafter remains higher than that of the general population [97, 120,121,122,123,124,125,126,127]. This effect of recency of fracture, sometimes termed imminent risk [126], is also dependent on age, sex and site of fracture [53]; (evidence level Ic). This complexity is being addressed by the development of optional post-FRAX algorithms to allow clinicians to explore the potential impact of fracture recency on the calculated probability of MOF and hip fracture (Table 1) [53]. The mechanism underlying imminent risk is not yet fully understood and no clinical risk factors have yet been identified for short term recurrent fractures that differ from those identified for fracture over a longer time horizon [78]. Few therapeutic studies have reported the recency of fracture in those patients whom they have recruited, though rapid clinical efficacy has been demonstrated within studies of zoledronate, risedronate, teriparatide, and romosozumab [115, 128, 129]; (evidence level Ib).
A NOGG threshold that characterises men and women at high and very high fracture risk has also been established using FRAX probabilities; very high risk is identified as a FRAX-based fracture probability that exceeds the intervention threshold by 60% (Figs. 1 and 2) [130]. It can be used to identify patients who likely require specialist referral for assessment of their osteoporosis (which should include DXA measurement of BMD), and further consideration of appropriate treatment strategies [117, 131]. The proportion of postmenopausal women at very high risk defined in this way rises from approximately 6% at age 50–54 to 36% at age 90 years or older. Numerical values for the probability thresholds are given in Table 4 for MOF and for hip fracture. An assessment algorithm is shown in Fig. 3. In patients with FRAX probabilities in the high-risk category, consideration of additional clinical risk factors (e.g., frequent falls, very low spine BMD; see Table 1) can also lead to redesignation from high to very high risk of fracture.
Management algorithm for the assessment of individuals at risk of fracture [130]. Those at very high risk should be treated and considered for referral to an osteoporosis specialist in secondary care; some may benefit from parenteral treatment (including first-line anabolic drug treatment, especially if multiple vertebral fractures). All individuals should be offered lifestyle advice. CRF, clinical risk factor
FRAX—practical considerations
The FRAX MOF probabilities are transferred automatically to the NOGG website, by clicking on the specified button on the FRAX results box. Where practitioners receive the results of a FRAX risk assessment for an individual patient without treatment guidance, the FRAX probabilities can also be entered manually onto the NOGG website; this page also captures additional information (age, sex, glucocorticoid exposure and finally, whether a femoral neck BMD has been included, in the FRAX estimates) so that the result can be automatically compared to the NOGG thresholds with appropriate guidance on treatment. Lack of integration of FRAX assessments and links to NOGG guidance in existing patient health record systems represents a barrier to effective fracture risk assessment (evidence IV).
The targeted use of BMD assessments with the NOGG strategy makes more efficient use of often limited resources than would DXA scanning of all individuals with risk factors [132]; (evidence level Ib). Historically it was thought that treatment should not be undertaken in women without initial BMD measurement, except in those with hip or vertebral fractures. This view arose after a post hoc analysis in 1998 suggested reduced efficacy of alendronate in patients with BMD T-scores above -2.5 [133]; (evidence level Ib). However, this approach is now outdated as many studies have since shown little or no interaction of BMD on the effectiveness of several agents, including bisphosphonates (e.g., zoledronate, denosumab, raloxifene, and teriparatide) [64, 134,135,136,137]; (evidence level Ib). Moreover, clinical risk factors are not independent of BMD and, when clinical risk factors alone are used in women age 70 years or more to identify patients at high fracture risk, BMD is approximately 1SD lower in the high-risk group compared with a low-risk group [138, 139]; (evidence level Ib). These findings indicate that the categorisation of patients at high fracture risk on the basis of FRAX without BMD mostly selects patients with low BMD and that the higher the fracture probability, the lower the BMD. Note that this does not preclude the use of DXA scanning if more widely available; in addition to providing the most accurate risk assessment, DXA provides a baseline measurement for treatment monitoring and also permits, again if available and indicated, detection of vertebral fractures using VFA. FRAX is not recommended as a tool to monitor treatment [140]; (evidence level IIb). However, the use of FRAX is appropriate to re-evaluate current fracture probabilities when considering a change in patient management; (evidence level IV).
Non-pharmacological management of osteoporosis
Recommendations
Postmenopausal women, and men age ≥ 50 years, with osteoporosis or who are at risk of fragility fracture are recommended the following:
-
1.
A healthy, nutrient-rich balanced diet (strong recommendation).
-
2.
An adequate intake of calcium (minimum 700 mg daily) is preferably achieved through dietary intake or otherwise by supplementation (strong recommendation).
-
3.
To consume vitamin D from foods be prescribed vitamin D supplements of at least 800 IU/day if they have identified vitamin D insufficiency or risk factors for vitamin D insufficiency. Those who are either housebound or living in residential or nursing care are more likely to require calcium and vitamin D supplementation to achieve recommended levels of intake (strong recommendation).
-
4.
A combination of regular weight-bearing and muscle-strengthening exercise, tailored according to the individual patient’s needs and ability (strong recommendation).
-
5.
Advice about smoking cessation if an individual is a smoker (strong recommendation).
-
6.
Advice to restrict alcohol intake to ≤ 2 units/day (strong recommendation).
-
7.
A falls assessment should be undertaken in all patients with osteoporosis and fragility fractures; those at risk should be offered exercise programmes to improve balance and/or that contain a combined exercise protocol (strong recommendation).
Dietary modification
A meta-analysis of observational studies examining different dietary patterns found a modest reduction in risk of low BMD and of hip fractures in subjects adhering to ‘healthy’ (high in fruit and vegetables, fish, poultry and whole grains) diets and a reduction in risk of low BMD in those with ‘milk/dairy’ diets. By contrast, those with a ‘meat/Western’ dietary pattern (high in processed and red meat, animal fat, refined sugar, and soft drinks) saw a modest increase in the risk of low BMD and hip fractures. However, population heterogeneity with the inclusion of subjects aged under 25 years in many dietary studies reduces generalisability [141]; (evidence level IIa). A randomised controlled trial of a ‘healthy diet’ consumed for 30 days, specifically a calcium-rich diet that emphasizes fruits, vegetables and low-fat dairy products (Dietary Approaches to Stop Hypertension (DASH)), resulted in a reduction in bone turnover [142]; (evidence level Ib).
Protein is an important constituent of bone and muscle tissue, and good dietary intake is necessary to maintain the health of the musculoskeletal system. Protein intakes higher than the recommended daily allowance (RDA) of 0.75 g/kg body weight/day are associated with higher BMD at the neck of femur and total hip in one RCT, and in observational studies, has been associated with a reduced risk of hip fractures[143, 144]; (evidence levels Ib and IIa); however, in a meta-analysis of 30 interventional studies, no significant effects of protein supplementation on BMD were seen[144]; (evidence level Ia). Post-operative protein supplementation in patients with a recent hip fracture has been shown to improve the subsequent clinical course by significantly lowering the rates of infection and duration of hospital stay [145]; (evidence level Ib).
Whilst there are inconsistencies in the evidence base for the associations between vegetarian and vegan diets and musculoskeletal health, consumption of a vegetarian or vegan diet has been associated with lower BMD at the lumbar spine and hip than an omnivore diet, and a vegan diet has been associated with higher fracture risk [146]; (evidence level IIa). A subsequent prospective cohort study of 65,000 people in the UK also identified lower BMD at the spine and hip in vegans and vegetarians, and higher hip fracture risk in vegans, attenuated in part by adjustment for calcium and/or protein intake [147]; (evidence level IIb).
Calcium and vitamin D
At every stage of life, adequate dietary intakes of key bone nutrients such as calcium and vitamin D contribute to bone health. The UK Reference Nutrient Intake per day of calcium is 700 mg for adults aged 19 years and older [148]. Dietary calcium calculators are available to assess intake, e.g., https://www.cgem.ed.ac.uk/research/rheumatological/calcium-calculator/. Whilst the Scientific Advisory Committee on Nutrition (SACN) recommends a reference nutrient intake (RNI) of 400 IU daily of vitamin D for adults of all ages [149], in the context of osteoporosis higher levels, specifically 800 up to 2,000 IU daily may be appropriate [150]; (evidence level IV).
Most randomised controlled trials of anti-resorptive and anabolic drugs have included co-administration of calcium and vitamin D supplements. There have been many randomised controlled trials of either calcium alone, vitamin D alone, or both in combination to examine whether the use of these supplements alone reduces fracture risk. With respect to combined calcium and vitamin D supplements, meta-analyses have reported a reduction in hip and non-vertebral fractures, and possibly also in vertebral fractures [151,152,153]; (evidence level Ia). Overall, there is little evidence that vitamin D supplementation alone reduces fracture incidence, although it may reduce falls risk [153, 154]; (evidence level Ib). However, it is important for patients taking antiresorptive and anabolic osteoporosis drug therapies to be vitamin D replete. In clinical practice, dietary sources of calcium are the preferred option and calcium (combined with vitamin D) supplementation should be targeted to those who do not get sufficient calcium from their diet and who are at risk of osteoporosis and/or fragility fracture, such as older adults who are housebound or living in residential or nursing care [152], and those with intestinal malabsorption e.g. due to chronic inflammatory bowel disease, or following bariatric surgery. Calcium and vitamin D supplements may increase the risk of kidney stones, but not the incidence of cardiovascular disease or cancer [155]; (evidence level Ia). Routine intermittent administration of large doses of vitamin D, e.g. ≥ 60,000 IU, is not advised, based on reports of an associated increased risk of fracture and falls [156, 157]; (evidence level Ia).
Exercise to improve or maintain bone density
Exercise has beneficial effects on BMD [158] (evidence level Ia); however, clear evidence for a reduction in fracture risk is wanting. The effect of exercise on different skeletal sites varies. Combination exercise programmes, which include weight-bearing and resistance strengthening exercise, are effective at reducing bone loss in the femoral neck and lumbar spine in post-menopausal women [158, 159]; (evidence level Ia). Similarly, upper body resistance exercise increases forearm bone mass [160]; (evidence level Ia). A meta-analysis of the effects of exercise interventions on BMD in men found only three studies and identified a significant but moderate improvement in BMD at the femoral neck and a trend towards increased BMD at the lumbar spine [161]; (evidence level Ia).
The effect of exercise varies with intensity and duration. Strengthening (resistance) exercise may be more effective if supervised. People at risk of falls, or with vertebral fractures, may need more specific advice and assessment before increasing exercise intensity [162]. The NOGG supports the Royal Osteoporosis Society Strong, Steady and Straight Expert Consensus Statement, which offers advice on intensity and duration and linked patient information videos and factsheets [162]. In people with osteoporosis, repetitive forced spinal forward flexion exercises should be undertaken with care as this specific movement may be associated with an increased risk of new vertebral fractures [163]; (evidence level Ia). However, in general people with osteoporosis can safely participate in exercise because the risk of serious adverse events is very low [163]; (evidence level Ia).
Falls interventions
The majority of non-vertebral fractures are preceded by a fall. Exercise can significantly reduce the risk of falls and, perhaps the risk of subsequent fractures, by maintaining or restoring muscle strength, balance and posture, improving confidence and reaction times. However, two recent large randomised controlled trials have not demonstrated an effect of multi-disciplinary interventions, targeted at falls, on fracture reduction, when combined with screening for falls risk in 70 [164, 165]; (evidence level Ib), a recent Cochrane review of falls prevention exercise programmes, and two previous meta-analyses demonstrated, albeit with low certainty, evidence of a reduction in fall-related fractures (or falls resulting in fractures) in those living in the community [159, 166, 167]; (evidence level Ia). Exercise interventions to reduce falls in people with osteoporosis and/or at high risk of falling have been found to be safe [168]; (evidence level Ia). Programmes that involve balance training and/or a combined exercise protocol are more effective in those who have risk factors for falling [166, 168]; (evidence level Ia). Combined exercise protocols may include resistance training, balance challenging, aerobic exercise and impact exercise. Interventions of 3 h per week or more are most effective [169]; (evidence level Ia). Interventions of short duration (less than 6 months) have been found to be effective, and good compliance with exercise interventions has been reported [168]; (evidence level Ia). Home safety interventions (best delivered by an occupational therapist) have been shown to reduce the risk of falls in people living in the community [170]; (evidence level Ia). Furthermore, whole-body vibration has been demonstrated to reduce fall rate but does not increase BMD [171]; (evidence level Ia).
Lifestyle measures
Other measures to improve bone health include optimisation of body mass index if under or overweight, stopping smoking and reducing alcohol intake. Smoking cessation has been demonstrated to reduce the risk of vertebral and hip fractures in women [172, 173]; (evidence levels Ilb and IIa). However, the risk of hip fracture was reduced in those who had stopped smoking, compared with current smokers, only after 5 years. Furthermore, pre-operative smoking cessation is associated with fewer post-operative complications [174]; (evidence level Ia). In men with previous alcohol dependence, BMD is significantly lower than in controls, but improves following 3–4 years of abstinence [175]; (evidence level IIa). The national guidelines recommend alcohol intake is limited to ≤ 2 units/day for women and men [176].
Pharmacological treatment options
Recommendations
-
1.
Fracture risk assessment, patient suitability and preference and cost-effectiveness should inform the choice of drug treatment. In most people at risk of fragility fracture, anti-resorptive therapy is the first-line option (strong recommendation).
Antiresorptive drug treatment
-
2.
Offer oral bisphosphonates (alendronate or risedronate) or intravenous zoledronate as the most cost-effective interventions. Alternative options include denosumab, ibandronate, hormone replacement therapy, raloxifene and strontium ranelate (strong recommendation).
-
3.
Offer intravenous zoledronate as a first-line treatment option following a hip fracture (strong recommendation).
-
4.
Before starting denosumab, ensure a long-term personalised osteoporosis management plan is in place and that both the patient and the primary care practitioner are made aware that denosumab treatment should not be stopped or delayed without discussion with a healthcare professional (strong recommendation).
-
5.
Avoid unplanned cessation of denosumab because it can lead to increased vertebral fracture risk, hence it must not be stopped without considering an alternative therapy (strong recommendation).
-
6.
If denosumab therapy is stopped, intravenous infusion of zoledronate is recommended 6 months after the last injection of denosumab, with subsequent monitoring of serum CTX guiding the timing of further treatment (strong recommendation). Where monitoring of serum CTX is not possible, consider a further intravenous infusion of zoledronate 6 months after the first dose of zoledronate (conditional recommendation).
-
7.
Limit the initiation of HRT for the treatment of postmenopausal osteoporosis to younger post-menopausal women (age ≤ 60 years) who have low baseline risk for adverse malignant and thromboembolic events (strong recommendation).
-
8.
Discuss continued use of HRT after the age of 60 years with the patient, with treatment based on an individual risk–benefit analysis (conditional recommendation).
Anabolic drug treatment
-
9.
Consider teriparatide or romosozumab as first-line treatment options in postmenopausal women at very high fracture risk, particularly in those with vertebral fractures (conditional recommendation).
-
10.
Consider teriparatide as a first-line treatment option in men age 50 years and older who are at very high fracture risk, particularly in those with vertebral fractures (conditional recommendation).
-
11.
Consider as second-line treatment options, teriparatide in postmenopausal women, and men age 50 years and older, and romosozumab in postmenopausal women, who are intolerant of bisphosphonate treatment, particularly in those with vertebral fractures (conditional recommendation).
-
12.
Following the approved duration of treatment with teriparatide or romosozumab (24 or 12 months respectively), initiate treatment with alendronate, zoledronate or denosumab without delay (strong recommendation).
-
13.
Consider raloxifene as an option for follow-on treatment after an anabolic drug in women (conditional recommendation).
Other treatments
-
14.
When other antiresorptive and anabolic treatments are contraindicated or not tolerated, strontium ranelate can be used to treat postmenopausal osteoporosis and men with severe osteoporosis, provided the risk–benefit in relation to cardiovascular and thromboembolic events is considered. Initiation by a specialist who is an expert in osteoporosis management is advised (strong recommendation).
-
15.
Offer calcium and/or vitamin D supplementation as an adjunct to anti-osteoporosis drug treatment, if dietary calcium is low and/or vitamin D insufficiency is a risk, respectively (strong recommendation).
-
16.
Treat vitamin D deficiency and insufficiency prior to initiation of parenteral anti-osteoporosis drug treatment, and alongside initiation of oral anti-osteoporosis drug treatment (strong recommendation).
Overview of treatment options
Drugs used in the management of osteoporosis can be considered under two broad headings based on their primary mode of action. Anti-resorptive drugs primarily inhibit osteoclastic bone resorption with later secondary effects on bone formation. Anabolic drugs primarily stimulate osteoblastic bone formation with variable effects on bone resorption. Most drugs fit into one or another category but romosozumab has a dual action, both stimulating bone formation and inhibiting bone resorption. Anti-resorptive drugs are much less expensive than anabolic drugs. It is important to consider the long-term management strategy for each patient initiated on osteoporosis treatment, as the timing of use of certain drugs is important; for example, teriparatide can only be used once in a lifetime, whilst denosumab requires careful consideration before initiation is given the difficulties in stopping treatment once it is started.
The drugs listed in Table 5 have been shown to reduce fragility fractures in postmenopausal women, and men where indicated, with osteoporosis [177] (evidence levels Ia and Ib). The efficacy of the drugs listed in Table 5 is well established for the prevention of vertebral fractures. Teriparatide and romosozumab are superior to risedronate and alendronate respectively at reducing vertebral fractures in high-risk postmenopausal women with osteoporosis. Most drugs listed in Table 5 have been shown to reduce hip fracture incidence, with the exception of ibandronate, calcitriol and raloxifene. Drugs listed in Table 5 (except calcitriol and raloxifene) have been shown to reduce the incidence of non-vertebral fractures.
At the time of writing decision is pending from NICE regarding the use of romosozumab in England, Wales and Northern Ireland; the initial decision from NICE is negative, but this is currently under consultation.
Primary and secondary care drug initiation
Oral and intravenous bisphosphonates, denosumab, raloxifene, calcitriol, and HRT can be initiated by primary or secondary care clinicians. If denosumab is initiated in 70, consultation with secondary care colleagues is advised given the need to have a long-term personalised osteoporosis management plan in place before denosumab is started, to enable denosumab, to be stopped in a managed way, as necessary. As calcitriol use is only supported by a grade IIa evidence base, its use is generally restricted to a select sub-group managed through secondary care. Strontium ranelate can be initiated by primary or secondary care clinicians, but if started in 70 should involve consultation with secondary care.
Secondary care drug initiation.
Teriparatide and romosozumab should be initiated by secondary care clinicians. In the UK both are provided via ‘home healthcare’ services, which also provide patient education.
Treatment sequence
Any patient stopping denosumab, romosozumab or teriparatide requires a sequential therapy strategy usually involving an anti-resorptive drug, which should be planned at the time the initial therapy is instigated to avoid a gap in treatment.
Specific drug options
Anti-resorptive drugs: bisphosphonates
Alendronate 70 mg once weekly by mouth is recommended for the treatment of women with postmenopausal osteoporosis (PMO), men with osteoporosis; glucocorticoid-induced osteoporosis (GIO), and the prevention of PMO and GIO. The 70 mg weekly dose is considered equivalent to the previously approved dose of 10 mg daily. In postmenopausal women with osteoporosis, alendronate has been shown to reduce vertebral, non-vertebral and hip fractures [178]; (evidence level Ib). Approval for the use of alendronate in men with osteoporosis and in men and women taking glucocorticoids was granted on the basis of BMD bridging studies [179, 180]; (evidence level Ib). Although the daily dose of alendronate (10 mg) is licenced for use in men, this is considered equivalent to the weekly dose (70 mg); (evidence level IV).
Common side-effects of alendronate include upper gastrointestinal symptoms, bowel disturbance, headaches and musculoskeletal pain. Alendronate should be taken after an overnight fast and at least 30 min before the first food or drink (other than water) of the day or any other oral medicinal products or supplementation (including calcium). Tablets should be swallowed whole with a glass of plain water (~ 200 ml) whilst the patient is sitting or standing in an upright position. Patients should not lie down for 30 min after taking the tablet. Alendronate is also available as 70 mg effervescent or soluble tablets, to be dissolved in a glass of plain water (≥ 120 ml).
Risedronate 35 mg once weekly by mouth is recommended for the treatment of PMO, men with osteoporosis; GIO and the prevention of GIO in women. The 35 mg weekly dose is considered equivalent to the previously approved dose of 5 mg daily. In postmenopausal women with osteoporosis, risedronate has been shown to reduce vertebral and non-vertebral fractures [181, 182]; (evidence level Ib). In a large population of older women, risedronate significantly decreased the risk of hip fractures, an effect that was greater in osteoporotic women [65]; (evidence level Ib). Approval for use of risedronate in men with osteoporosis and in postmenopausal women taking glucocorticoids was granted on the basis of BMD bridging studies [183,184,185]; (evidence levels Ib).
Common side-effects include upper gastrointestinal symptoms, bowel disturbance, headache and musculoskeletal pain. Risedronate should be taken after an overnight fast and at least 30 min before the first food or drink (other than water) of the day or any other oral medicinal products or supplementation (including calcium). Tablets should be swallowed whole with a glass of plain water (≥ 120 ml) whilst the patient is sitting or standing in an upright position. Patients should not lie down for 30 min after taking the tablet.
Ibandronate 150 mg once monthly by mouth or 3 mg as a prefilled intravenous injection (usually given as a 15–30-s push via butterfly cannula) every 3 months is recommended for the treatment of postmenopausal women with osteoporosis. The 150 mg monthly dose and 3 mg 3-monthly intravenous dose are considered equivalent to the following doses: 2.5 mg daily by mouth for the treatment of PMO. In postmenopausal women with osteoporosis, ibandronate 2.5 mg daily has been shown to reduce vertebral fracture incidence [186]; (evidence level Ib). In a post hoc analysis of women at high fracture risk (with a femoral neck BMD T-score below − 3.0), a significant reduction in non-vertebral fractures was shown [187]; (evidence level Ib). No data are available to show the efficacy of hip fracture risk reduction. Approval for the oral 150 mg once monthly and 3 mg intravenously every 3 months formulations was granted on the basis of BMD bridging studies [188, 189]; (evidence level Ib).
Common side-effects with oral preparation include upper gastrointestinal side-effects and bowel disturbance. Intravenous administration may be associated with an acute-phase reaction, characterised by an influenza-like illness; this is generally short-lived and typically occurs only after the first injection. Oral ibandronate should be taken after an overnight fast and 1 h before the first food or drink (other than water) of the day, or any other oral medicinal products or supplementation (including calcium). Tablets should be swallowed whole with a glass of plain water (180 to 240 ml) whilst the patient is sitting or standing in an upright position. Patients should not lie down for 1 h after taking the tablet.
Zoledronate 5 mg once yearly by intravenous infusion (as 5 mg/100 ml infusion given over a minimum of 15 min via an intravenous cannula) is recommended for the treatment of PMO, men with osteoporosis and men and postmenopausal women with GIO. In postmenopausal women with osteoporosis, zoledronate 5 mg once yearly has been shown to reduce the incidence of vertebral, non-vertebral and hip fractures [190]; (evidence level Ib). Approval for use of zoledronate in men with osteoporosis and in men and women taking glucocorticoids was granted on the basis of BMD bridging studies [191, 192]; (evidence level Ib). When given shortly after hip fracture, men and women given zoledronate 5 mg annually had had fewer clinical fractures and lower mortality 3 years later [129]; (Evidence level Ib). When given (without calcium supplementation) every 18 months to women with osteopenia, there were fewer vertebral and non-vertebral fractures [137, 193]; (evidence level Ib). A lower although non-significant decrease in mortality in fracture-free women, fewer breast cancers and fewer non-breast cancers were also reported as secondary outcomes by the end of the 6-year study.
Common side-effects include an acute phase reaction usually only after the first infusion [194], which can be ameliorated by co-administration of paracetamol. Glomerular filtration rate (eGFR) should be calculated prior to initiation of treatment and caution advised for recipients at risk of kidney failure; monitoring for any increase in serum creatinine or reduction in eGFR. The MHRA recommends the use of creatinine clearance instead of eGFR to inform treatment decisions in those aged over 75 years and/or with BMI < 18 or > 40 kg/m2. An increase in symptomatic atrial fibrillation, reported as a serious adverse event, was seen in the main phase III trial [190]; (evidence level Ib).
Contraindications and special precautions for the use of bisphosphonates
Oral and intravenous bisphosphonates are contraindicated in patients with hypocalcaemia, hypersensitivity to bisphosphonates, in women who are pregnant or lactating. Oral bisphosphonates are contraindicated in people with abnormalities of the oesophagus that delay oesophageal emptying such as stricture or achalasia, and inability to stand or sit upright for at least 30–60 min. They should be used with caution in patients with other upper gastrointestinal disorders. Zoledronate and risedronate are contraindicated in severe renal impairment (GFR ≤ 35 ml/min for zoledronate and ≤ 30 ml/min for risedronate), whilst alendronate and ibandronate are cautioned against (GFR ≤ 35 ml/min for alendronate and ≤ 30 ml/min for ibandronate). Pre-existing hypocalcaemia must be investigated and, where due to vitamin D deficiency, treated with vitamin D (e.g., 100,000 to 300,000 IU orally as a loading dose in divided doses) before zoledronate treatment is initiated. Rare adverse effects of long-term bisphosphonate treatment including osteonecrosis of the jaw and atypical femoral fractures are addressed later.
Anti-resorptive drugs: denosumab
Denosumab is a fully humanised monoclonal antibody against Receptor Activator of Nuclear factor Kappa B Ligand (RANKL), a major regulator of osteoclast development and activity. It is approved for the treatment of PMO and men at increased fracture risk, for the treatment of bone loss associated with hormone ablation in men with prostate cancer at increased fracture risk, and for the treatment of bone loss associated with long term systemic glucocorticoid therapy in adults at risk of fragility fracture [195]; (evidence level Ib). Denosumab is given as a subcutaneous injection of 60 mg once every 6 months. It has been shown to reduce the incidence of vertebral, non-vertebral and hip fractures in postmenopausal women with osteoporosis [196] and safety and efficacy are maintained over 10 years of treatment [197]; (evidence level Ib). Approval for its use in men with osteoporosis was granted on the basis of a BMD bridging study [198]; (evidence level Ib).
Denosumab is contraindicated in patients with hypocalcaemia or with hypersensitivity to any of the constituents of the formulation. Its use is not recommended in pregnancy or in those aged < 18 years. Hypocalcaemia, as a side-effect of denosumab treatment, increases with the degree of renal impairment; patients should be advised to report symptoms of hypocalcaemia. Pre-existing hypocalcaemia must be investigated and, where due to vitamin D deficiency, treated with vitamin D (e.g., 100,000 to 300,000 IU orally as a loading dose in divided doses) before denosumab treatment is initiated. Adequate intake of calcium and vitamin D is important in all patients, especially those with severe renal impairment. The SPC states all patients should have calcium checked prior to each dose. In patients predisposed to hypocalcaemia (e.g., patients with a creatinine clearance < 35 ml/min), serum calcium levels should also be checked within 2 weeks after the initial dose [199]. Side-effects include skin infection, predominantly cellulitis, eczema, hypocalcaemia, and flatulence. Rare adverse effects of denosumab include osteonecrosis of the jaw and atypical femoral fractures and are addressed later.
Denosumab cessation leads to rapid reductions in BMD and elevations in bone turnover to levels above those seen before treatment initiation [200,201,202]; (evidence level Ib). Patients who discontinue denosumab have an increased risk of sustaining multiple vertebral fractures. In a post hoc analysis of the FREEDOM study and its extension, women discontinuing denosumab had an increased rate of vertebral fracture over an average of 3–6 months since the last denosumab injection was due. Of those patients who sustained vertebral fractures, 60.7% sustained multiple fractures compared to 38.7% of those discontinuing placebo [203, 204]; (evidence level Ib). The increase in vertebral fracture risk following cessation of denosumab therapy emphasises the need to consider continued treatment with an alternative anti-resorptive drug following denosumab withdrawal. An intravenous infusion of 5 mg of zoledronate, 6 months after the last denosumab injection, reduces subsequent bone loss [205,206,207,208,209], although this effect is not seen in all patients and may not be maintained beyond one year, particularly in those who have had more than 3 years of denosumab treatment [210] (evidence levels IIa and IIb). Monitoring bone turnover markers at 3 and 6 months post zoledronate infusion can help guide the timing of subsequent infusions. Where bone turnover markers are not available, a second infusion of zoledronate after 6 months has been proposed [211]; (evidence level IV). Oral alendronate 70 mg once weekly, was shown to maintain BMD for 12 months in most patients following one year of denosumab therapy, although significant bone loss occurred in a minority [212]; (evidence level IIa). Given the difficulties in stopping denosumab treatment, particularly careful consideration is needed before starting denosumab in younger postmenopausal women, and men.
Anti-resorptive drugs: hormone-replacement therapy
Hormone-replacement therapy (HRT) comprises a large number of oestrogen formulations or oestrogen plus progestogen combinations, some of which are approved for the prevention of osteoporosis in postmenopausal women at risk of fragility fracture. Conjugated equine oestrogens 0.625 mg daily ± 2.5 mg/day of medroxyprogesterone acetate has been shown to reduce vertebral, non-vertebral and hip fracture risk in postmenopausal women not selected on the basis of low bone density or high fracture risk [213, 214]; (evidence level Ib). The benefit-risk balance of HRT use in postmenopausal women within the age range 53–79 years, was reviewed in 2017. Women using estrogen-only therapy compared with placebo had a significantly lower risk of fractures but significantly higher risk of gall bladder disease, stroke, venous thromboembolism, and urinary incontinence. Women using oestrogen plus progestin in combination compared with placebo had a significantly lower risk of fractures but had a significantly higher risk of invasive breast cancer, probable dementia, gallbladder disease, stroke, urinary incontinence, and venous thromboembolism [215]; (evidence level Ib). A more recent narrative review concluded that overall, the benefit-risk profile supports the use of HRT in the management of osteoporosis in women < 60 years old, who have recently (within 10 years) become menopausal, who have menopausal symptoms and have low baseline risk for adverse events [216]; (evidence level IIa).
Anti-resorptive drugs: calcitriol
Calcitriol (1,25-dihydroxyvitamin D3) is the active form of vitamin D and is approved for the treatment of established postmenopausal osteoporosis in an oral dose of 0.25 µg twice daily. It acts mainly by inhibiting bone resorption. It has been shown to reduce vertebral fracture risk in postmenopausal women with osteoporosis but effects on non-vertebral and hip fractures have not been demonstrated [217]; (evidence level IIb). It is contraindicated in patients with hypercalcaemia or with metastatic calcification. Because calcitriol can cause hypercalcaemia and/or hypercalciuria, serum calcium and creatinine levels should be monitored at 1, 3, and 6 months after starting treatment and at 6 monthly intervals thereafter.
Anti-resorptive drugs: raloxifene
Raloxifene is a selective oestrogen receptor modulator and inhibits bone resorption. It is approved for the treatment and prevention of osteoporosis in postmenopausal women. Raloxifene has been shown to reduce vertebral fracture risk but the reduction in non-vertebral and hip fractures has not been demonstrated [218]; (evidence level Ib). Raloxifene is taken orally as a single daily 60 mg dose and may be taken at any time without regard to meals. Raloxifene is contraindicated in women with child-bearing potential, unexplained uterine bleeding, severe hepatic or renal impairment and in women with a history of venous thromboembolism. Side-effects include leg cramps, oedema and vasomotor symptoms. There is a small increase in the risk of venous thromboembolism, mostly within the first few months of treatment and a small increase in the risk of fatal stroke has been reported [219], (evidence level IIa) such that it should be used with caution in women with a history of stroke or with risk factors for stroke disease. In the phase III trials, women treated with raloxifene had a significantly decreased risk of developing breast cancer [220]; (evidence level Ib).
Other drugs: strontium ranelate
Strontium ranelate is taken in a dose of 2 g once at night by mouth as a suspension of granules stirred in water, at least 2 h after food and/or consumption of calcium-containing products. As an alkaline earth metal (closely related to calcium) it substitutes for calcium within hydroxyapatite. Its mode of action is not completely understood but the evidence suggests it has weak anti-resorptive effects whilst maintaining bone formation. In postmenopausal women with osteoporosis, strontium ranelate 2 g daily has been shown to reduce the incidence of vertebral and non-vertebral fractures [221, 222]; (evidence level Ib). Fewer hip fractures were reported in a post hoc analysis of women at high risk of hip fracture (i.e., age ≥ 74 years with a femoral neck BMD T-score ≤ − 2.5). Approval for its use in men with osteoporosis was granted on the basis of a BMD bridging study [223]; (evidence level Ib). Common side effects include nausea and diarrhoea. There was a significant increase in venous thromboembolism in the phase III trials [224]. Contraindications include previous myocardial infarction, stroke, or venous thromboembolism as a post hoc pooled safety analysis showed significant increases in myocardial infarction and ‘nervous system disorders’ including cerebrovascular disease was observed in patients taking strontium ranelate compared to placebo [225]. The manufacturer advises against use when the eGFR is < 30 ml/ml. The higher atomic number of strontium compared with calcium artefactually increases BMD when incorporated into the bone matrix [226]. When strontium ranelate is stopped, this effect is slow to resolve with implications for future BMD monitoring.
Anabolic drugs: teriparatide (recombinant human parathyroid hormone [PTH] 1–34)
When administered intermittently, teriparatide has anabolic skeletal effects which are most marked in trabecular bone. Teriparatide is approved for the treatment of osteoporosis in postmenopausal women and in men at risk of fragility fracture, and osteoporosis is associated with systemic glucocorticoid therapy in women and men at risk of fragility fracture. Teriparatide is given as a subcutaneous injection in a dose of 20 µg/day. The duration of treatment is limited to 24 months.
Teriparatide is contraindicated in patients with hypercalcaemia, metabolic bone diseases other than osteoporosis and osteogenesis imperfecta, severe renal impairment, a malignant disease affecting the skeleton, prior radiation to the skeleton, and in women who are pregnant or lactating. Teriparatide should be used with caution in patients with moderate renal impairment. PTH levels need to be normal to initiate teriparatide; hence, levels should be checked even with normocalcaemia. Side effects include headache, nausea, dizziness, postural hypotension, and leg pain. Slight and transient elevations of serum calcium may occur following teriparatide injection.
Teriparatide has been shown to reduce vertebral and non-vertebral fractures in postmenopausal women with osteoporosis [227]; (evidence level Ib). No primary efficacy end-point data are available for hip fracture incidence, but systematic review and meta-analysis level evidence has shown an OR for hip fracture risk of 0.44 (95% CI: 0.22, 0.87; p = 0.019) in patients treated with teriparatide compared with placebo, when considering hip fracture as a safety endpoint. No significant benefit was seen on upper limb fractures [228]; (evidence level Ia). These findings were further supported by a network meta-analysis of a similar list of RCTs, which reported a HR of 0.35 (95% CI: 0.15, 0.73) for hip fracture in patients treated with teriparatide compared with placebo [229]; (evidence level Ia). Approval for teriparatide use in men with osteoporosis and in men and women with glucocorticoid-induced osteoporosis was granted on the basis of BMD bridging studies [230, 231]; (evidence level Ib). Teriparatide biosimilars are now available which is expected to improve the cost-effectiveness of the use of generic teriparatide.
Anabolic drugs: romosozumab
Romosozumab is a humanised monoclonal antibody that binds to and inhibits sclerostin. It has a dual action, stimulating bone formation and inhibiting bone resorption and is approved for the treatment of severe osteoporosis in postmenopausal women at very high risk of fracture. It is currently not approved for use in men. It is given as a subcutaneous injection in a dose of 210 mg (administered as two subcutaneous injections of 105 mg each) once monthly. The duration of treatment is limited to 12 months.
In postmenopausal women with osteoporosis who received romosozumab 210 mg or placebo subcutaneously once monthly for 12 months followed by denosumab 60 mg subcutaneously in both groups for 12 months, new vertebral fractures and clinical fractures were significantly reduced in women treated with romosozumab when compared to placebo at 12 months, and at 24 months vertebral fracture rates were significantly lower in women treated with romosozumab during the first 12 months [114]; (evidence level Ib). In a comparator-controlled study in postmenopausal women with severe osteoporosis subcutaneous romosozumab 210 mg once monthly for 12 months followed by oral alendronate 70 mg once weekly for 12 months was compared against alendronate 70 mg once weekly for 24 months) [115]. New vertebral, non-vertebral, clinical and hip fractures were all significantly lower in women treated with romosozumab followed by alendronate than in those treated with alendronate alone (evidence level Ib). Significantly greater risk reduction in new vertebral and clinical fractures was seen for romosozumab vs. alendronate at 12 months. A significantly higher incidence of cardiovascular events was seen in the romosozumab group compared to the alendronate group [114]; (evidence level Ib).
Romosozumab is contraindicated in patients with hypocalcaemia, hypersensitivity to any of the constituents of the formulation, or a history of myocardial infarction or stroke. When determining whether to use romosozumab for an individual patient, both fracture and cardiovascular risk (based on risk factors) over the next year need to be considered. Transient hypocalcaemia has been observed in patients receiving romosozumab. Hypocalcaemia should be corrected prior to initiation of treatment and patients should be adequately supplemented with calcium and vitamin D. Patients with severe renal impairment or on dialysis are at increased risk of developing hypocalcaemia. Osteonecrosis of the jaw and atypical femoral fractures have been very rarely reported with romosozumab use.
Drug treatment for patients with very high fracture risk
Two randomised comparator-controlled studies in postmenopausal women with severe osteoporosis have demonstrated superior anti-fracture efficacy of skeletal anabolic agents versus anti-resorptive drugs. Subcutaneous romosozumab 210 mg once monthly resulted in a significantly greater reduction of vertebral, non-vertebral, clinical and hip fractures at 24 months (risk reduction of 48%, 19%, 27% and 38% respectively) and significantly greater risk reduction in new vertebral and clinical fractures at 12 months when compared to oral alendronate 70 mg once weekly. In the VERtebral fracture treatment comparisons in Osteoporotic women (VERO) study, subcutaneous teriparatide, 20 µg once daily, was associated with significantly fewer new vertebral and clinical fractures than oral risedronate, 35 mg once weekly (56% and 52% respectively) after 2 years of treatment [232]; (evidence level Ib). These studies provide the rationale for considering teriparatide or romosozumab as a first-line treatment option in postmenopausal women at a very high risk of fracture. Comparator studies of anti-resorptive and anabolic agents have not been reported in men.
Following discontinuation of treatment with either teriparatide or romosozumab, bone turnover increases and there is a fall in BMD. Since the maximum permitted duration of treatment with teriparatide is 24 months and with romosozumab 12 months, sequential therapy with anti-resorptive drugs is required to maintain their beneficial skeletal effects. Both alendronate and denosumab have been shown to maintain and increase BMD at the spine and hip following either teriparatide or romosozumab therapy [115, 233,234,235,236]. In the FRAME extension study, the beneficial effects of 12 months romosozumab therapy on vertebral and non-vertebral fracture risk were maintained when followed by 24 months of denosumab treatment [237]; (evidence level IIb).
When women are switched from oral bisphosphonates to teriparatide or romosozumab, there is attenuation of the increase in spine and hip BMD compared to when these agents are used in treatment-naïve individuals. This blunting effect is greater for teriparatide than romosozumab, especially at the hip [238, 239]; (evidence level IIb). The impact of these effects, if any, on fracture risk is unknown. In women previously treated with denosumab, switching to teriparatide is associated with transient bone loss in the spine and greater and longer-lasting bone loss in the hip [234]. When romosozumab is given following denosumab therapy, there is attenuation of the BMD increase at the spine and hip [232, 240]; (evidence level IIb). The impact of these effects, if any, on fracture risk is unknown.
Duration and monitoring of bisphosphonate treatment
Osteoporosis is a long-term condition for which there is currently no cure, therefore life-long treatment and monitoring to prevent fractures is often required.
Recommendations
-
1.
Plan to prescribe oral bisphosphonates (alendronate, ibandronate and risedronate) for at least 5 years and then re-assess fracture risk. Longer durations of treatment, for at least 10 years, are recommended in the following men and women (strong recommendation) (see Fig. 4):
-
Age ≥ 70 years at the time that the bisphosphonate is started
-
Who have a previous history of a hip or vertebral fracture(s)
-
Treated with oral glucocorticoids ≥ 7.5 mg prednisolone/day or equivalent
-
Who experience one or more fragility fractures during the first 5 years of treatment (if treatment is not changed).
-
-
2.
Plan to prescribe intravenous bisphosphonate (i.e., zoledronate) for at least 3 years and then re-assess fracture risk. Longer durations of treatment, for at least 6 years, are recommended in the following men and women (strong recommendation) (see Fig. 5):
-
Age ≥ 70 years at the time that the bisphosphonate is started
-
Who have a previous history of a hip or vertebral fracture(s)
-
Treated with oral glucocorticoids ≥ 7.5 mg prednisolone/day or equivalent
-
Who experience one or more fragility fractures during the first 3 years of treatment (if treatment is not changed).
-
-
3.
If a new fracture occurs after bisphosphonate treatment is discontinued, reassess using FRAX and restart treatment (strong recommendation).
-
4.
If bisphosphonate treatment is discontinued and no new fracture occurs, reassess using FRAX after 18 months for risedronate and ibandronate, 2 years for alendronate, and 3 years for zoledronate to inform whether treatment should be restarted (strong recommendation).
Bisphosphonate therapy is associated with rare but serious adverse events, notably atypical femoral fractures (AFFs) and osteonecrosis of the jaw (ONJ). Defining the optimal duration of bisphosphonate therapy attempts to ensure that the benefit in fracture risk reduction outweighs the small risk of AFFs and ONJ at all time points through patient management. Bisphosphonates are retained the long term in bone allowing the beneficial effects to persist for some time after cessation of treatment administration. This has raised the possibility that some patients may benefit from a period off treatment to restore the benefit/risk balance [241]; (evidence level IIa), in which treatment is stopped after some years and the need for the reinstitution of therapy is subsequently reassessed. Treatment review in patients taking bisphosphonates is therefore critical [242] and each patient must be assessed individually to assess relative risks and benefits; there is no standard policy for ‘all patients’ [204]; (evidence level IIa). Because pivotal clinical trials have mostly been limited to a duration of three years, recommendations for longer-term use and for pauses in treatment are based on limited evidence from extension studies in postmenopausal women [243, 244]; (evidence level IIa). There is currently no evidence on which to base specific recommendations for men.
Withdrawal of bisphosphonate treatment is associated with decreases in BMD and increased bone turnover after 2–3 years for alendronate [245, 246]; (evidence level Ib), and 1–2 years for ibandronate and risedronate [247, 248]; (evidence level Ib). In the case of zoledronate, withdrawal after 3 years’ treatment is associated with only a small decrease in BMD after a further 3 years without treatment [249]; (evidence level Ib). Comparison between the offset of alendronate and zoledronate at 3 years showed alendronate-treated patients had greater reductions in total hip BMD and greater rises in PINP, despite a longer treatment exposure with alendronate, supporting a more rapid offset of drug effect than with zoledronate [250]; (evidence level IIb).
In the Fracture Intervention Trial Long-term Extension study of alendronate (FLEX), there were significantly fewer clinical vertebral fractures in women previously treated with alendronate for 5 years who continued with alendronate for five more years than in those assigned to placebo after 5 years of alendronate [246]; (evidence level Ib). In the Health Outcomes and Reduced Incidence with Zoledronate Once Yearly (HORIZON) study extension, the risk of morphometric vertebral fractures was significantly lower in women continuing on zoledronate for 3 years after the initial 3 years of therapy when compared to those switched to placebo [249]; (evidence level Ib). Post-hoc analyses from the alendronate and zoledronate extension studies suggest that women most likely to benefit from long-term bisphosphonate therapy are those with low hip BMD (T-score < − 2.0 in FLEX and ≤ − 2.5 in HORIZON), those with a prevalent vertebral fracture and those who sustained one or more incident fractures during the initial 3 or 5 years of treatment [71]; (evidence level Ib). Older age was also associated with increased fracture risk after discontinuation of alendronate therapy [251]; (evidence level Ib).
Reassessment of fracture risk in individuals on osteoporosis drug treatment
Recommendations
-
5.
Review treatment adherence in men and women who sustain a fragility fracture whilst on drug treatment, (poor adherence is when less than 80% of treatment has been taken correctly) and investigate for secondary causes of osteoporosis (strong recommendation).
-
6.
Fracture risk assessment in patients receiving drug treatment should be performed using FRAX with BMD, with arithmetic adjustments to FRAX probabilities to take account of additional clinical risk factors. If the FRAX-derived fracture probability exceeds the intervention threshold drug treatment should be continued (strong recommendation).
-
7.
If biochemical markers of bone turnover indicate relapse from suppressed bone turnover and/or BMD has decreased following bisphosphonate withdrawal, consider resumption of drug treatment (conditional recommendation).
-
8.
After 10 years of bisphosphonate treatment, patient management should be considered on an individual basis (conditional recommendation).
Stopping osteoporosis treatment, be it with bisphosphonate or denosumab, is associated with an increased risk of fragility fracture, such that routine cessation of anti-resorptive therapy (so-called drug holidays) is not supported by a review of the evidence [204]; (evidence level IIa). Reassessment of fracture risk in treated individuals can be performed using FRAX with femoral neck BMD [140]; (evidence level IIb). The NOGG intervention thresholds can then be used to guide the decision as to whether treatment can be stopped for a period of time (Figs. 4 and 5). Whereas FRAX cannot be used to assess treatment response [140]; (evidence level IIb) it does have a role in reassessing current fracture risk to determine the need to continue or to discontinue treatment. Detection of the offset of drug effect, using BMD and bone turnover changes, potentially provides information to influence clinical management. However, there are presently no definitive data that link a potential threshold change in BMD or bone turnover markers during drug offset to clinically meaningful changes in fracture risk.
Rare adverse effects of long-term bisphosphonate and denosumab treatment
Recommendations
-
9.
During bisphosphonate or denosumab therapy, encourage all patients to maintain good oral hygiene, receive routine dental check-ups, and report any oral symptoms such as dental mobility, pain, or swelling (strong recommendation).
-
10.
In those with the severe dental disease who require bisphosphonate or denosumab treatment, timely dental review and dental treatment by an appropriately experienced dental surgeon should be pursued before drug administration, bearing in mind drug treatment should be initiated as soon as possible after a fragility fracture; a multi-disciplinary team (MDT) approach to discuss individual needs is encouraged (conditional recommendation).
-
11.
During bisphosphonate or denosumab treatment, although ideally, patients should minimise invasive dental procedures where possible, if indicated they can be carried out safely and successfully in most patients. When dental procedures are required, there are no data available to show whether treatment discontinuation reduces the risk of ONJ. Clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment, ensuring patients continue to access routine dental care (conditional recommendation).
-
12.
During bisphosphonate or denosumab therapy, advise patients to report any unexplained thigh, groin or hip pain and if such symptoms develop, the femur should be imaged (by full-length femur X-ray, isotope scanning or MRI) (strong recommendation).
-
13.
If an AFF is identified, image the contralateral femur (strong recommendation).
-
14.
All patients who develop an AFF should be referred to an osteoporosis specialist to guide the management of future bone health (strong recommendation).
-
15.
In patients who develop an AFF, discontinue bisphosphonate or denosumab treatment (conditional recommendation).
Osteonecrosis of the jaw (ONJ) occurs only very rarely in patients receiving bisphosphonate or denosumab therapy for osteoporosis. The estimated incidence in those receiving bisphosphonates is 10–100/100,000 person-years of exposure in clinical trials. Risk factors for ONJ include poor oral hygiene, dental disease, dental interventions, smoking, cancer, chemotherapy and/or glucocorticoid therapy [252, 253]; (evidence level IIa). The incidence of ONJ is substantially greater with the higher doses of bisphosphonates or denosumab that are used to treat patients with skeletal metastases. The Scottish Dental Clinical Effectiveness Programme has produced guidance on oral health management in patients taking anti-resorptive medication [254]. Osteonecrosis of the external auditory canal after bisphosphonate treatment has been described very rarely in case reports, with patients presenting with ear symptoms including chronic ear infections. Possible risk factors include steroid use and chemotherapy and/ or local risk factors such as infection or trauma. [255]; (evidence level IV).
Atypical femoral fractures (AFF), mainly of the subtrochanteric and diaphyseal regions of the femoral shaft, have been reported rarely in patients taking bisphosphonates or denosumab for osteoporosis. Asian race, femoral bowing and glucocorticoid use have been identified as risk factors [256]. In a recent review by the ASBMR Task Force on the management of osteoporosis in patients on long-term bisphosphonates, a systematic search of the literature revealed that the absolute risk was consistently low, ranging between 3.2 and 50 cases/100,000 person-years of exposure [257, 258]; (evidence level IV). This estimate appeared to double with prolonged duration of BP use (> 3 years, median duration 7 years), and declined with discontinuation [257, 258]; (evidence level IV), [259]; (evidence level IIa). In a nationwide cohort study from Denmark, use of alendronate in excess of 10 years was associated with a 30% lower risk of hip fracture and no increase in the risk of fractures of the subtrochanteric femur and femoral shaft, supporting an acceptable risk–benefit balance in terms of fracture outcomes [260]; (evidence level IIb). Atypical femoral fractures are often bilateral, associated with prodromal pain and tend to heal poorly. Prodromal pain can be felt in the thigh, groin or hip for days, weeks, or months before fracture. Discontinuation of bisphosphonate or denosumab therapy is advised in patients who develop an atypical fracture, weight-bearing activity should be restricted, adequate calcium and vitamin D should be ensured, and alternative treatment options considered where appropriate. Surgical treatment with intramedullary nailing is often recommended [257, 258]; (evidence level IV).
There is a lack of good quality evidence on the medical management of bone health following an AFF. However, a recent international expert consensus document supported by a systematic review proposed practical measures to help in patient management [261]; (evidence level IV). Following an AFF, if the risk of fragility fracture is low, further pharmacological bone treatments can be avoided. If fracture risk is high, and bilateral surgical fixation of fractures has been performed, consider the use of teriparatide. If unilateral or no surgical intervention has taken place, consider teriparatide, romosozumab, raloxifene, or HRT. The potential utility of teriparatide as an adjunct to healing following AFF has been examined. There is no evidence that teriparatide enhances the healing of AFFs, but limited data show a tendency towards faster healing in surgically managed AFFs (complete and incomplete). However, in AFFs managed conservatively, there was no suggestion of improved fracture healing with teriparatide [261]; (evidence level IV). The benefits versus risks of using bisphosphonates or denosumab after AFF should be carefully examined if these options are considered, taking into consideration prior unilateral or bilateral nailing, use of an anabolic agent post AFF, together with the overall clinical situation and fracture risk (evidence level IV).
Glucocorticoid-induced osteoporosis
Recommendations
-
16.
Because bone loss and increased fracture risk occur early after initiation of oral glucocorticoids, bone-protective treatment should be started in the following people, at the same time as glucocorticoid therapy without waiting for bone density assessment, which should follow later (strong recommendations):
-
a)
Anyone with a prior fragility fracture,
-
b)
Women age ≥ 70 years,
-
c)
Postmenopausal women, and men aged ≥ 50 years, prescribed high doses of glucocorticoids, i.e., ≥ 7.5 mg/day of prednisolone or equivalent over 3 months (N.B., this is equivalent to ≥ 30 mg/day of prednisone for 4 weeks over 3 months)
-
d)
Postmenopausal women, and men aged ≥ 50 years, with a FRAX probability of major osteoporotic fracture or of hip fracture exceeding the intervention threshold.
-
a)
-
17.
Oral bisphosphonates (alendronate or risedronate) or intravenous zoledronate are the most cost-effective first-line drug options for bone protection. Denosumab is an alternative option. Teriparatide can be a first-line drug option in those at very high fracture risk (strong recommendation).
-
18.
Adequate calcium intake should be achieved through dietary intake if possible, with the use of supplements if necessary. An adequate vitamin D status should be maintained, using supplements if required (strong recommendation).
-
19.
If glucocorticoid therapy is stopped, withdrawal of bone-protective therapy may be considered at the same time, provided on re-assessment of fracture risk using FRAX, the probabilities of both major osteoporotic fracture and of hip fracture lie below the intervention threshold (strong recommendation).
-
20.
If glucocorticoids are continued long term, bone protection should be maintained in the majority of cases (strong recommendation).
Patients starting medium or low dose oral glucocorticoid therapy who have a FRAX probability near to, but below the intervention threshold, should have FRAX with BMD reassessed 12–18 months after starting glucocorticoid therapy (conditional recommendation).
Bone protective therapy may be appropriate in some premenopausal women and younger men, particularly in individuals with a previous history of fracture, or those receiving high doses of glucocorticoids (≥ 7.5 mg/day of prednisolone or equivalent over 3 months). Caution is advised when prescribing drug treatment in women of childbearing age. Referral of complex cases to secondary care is often necessary. Although guidance on the prevention and management of glucocorticoid-induced osteoporosis has been developed in many countries, there is evidence that in the UK osteoporosis risk assessment and management are still inadequate in long-term users of oral glucocorticoids [262]; (evidence level IIIb). Bone loss and increased fracture risk occur rapidly after initiation of oral glucocorticoid therapy and increase with the dose of glucocorticoids [56, 263]. The increase in fracture risk is seen for vertebral and non-vertebral fractures, including hip fractures, and is partially independent of BMD [57]; (evidence level Ia).
Approval for the use of bone protective therapy to prevent osteoporosis in people receiving oral glucocorticoids was based mainly on BMD bridging studies carried out as part of phase III randomised controlled trials with bisphosphonates [180, 185, 192, 264, 265]. Subsequently, approval has been given for denosumab using the same methodology [195]. Fracture prevention has not been considered as an efficacy end-point in most trials. However, although not a primary end-point, in an 18-month randomised controlled trial extended to 36 months comparing teriparatide with alendronate significantly fewer subjects in the teriparatide group had vertebral fractures compared with the alendronate arm [231], but with no benefit on non-vertebral fractures. This protection against vertebral fractures was confirmed in a recent metanalysis which showed that co-prescription of teriparatide, alendronate, risedronate or denosumab with glucocorticoids could reduce the incidence of vertebral fractures, with further evidence of a reduction in non-vertebral fracture rates with alendronate or teriparatide (Table 6) [266]; (evidence levels Ia and Ib).
Considering the increased fracture risk associated with higher glucocorticoid doses, FRAX assessment provides fracture probabilities based on both an average dose of prednisolone (2.5–7.5 mg/day or its equivalent), and a higher dose (≥ 7.5 mg/day or its equivalent). Individuals taking an average dose of prednisolone < 2.5 mg/day will have lower fracture risk, and the average adjustments over all ages in postmenopausal women, and men aged ≥ 50 years, are shown in Table 7 [83]; (evidence level IIb). For very high doses of glucocorticoids, i.e., > 20 mg/day prednisolone or its equivalent, greater upward adjustment of fracture probability is required [56]; (evidence level IIa).
When the UK FRAX model is used and the glucocorticoid box is filled, 2 points appear on the NOGG graphs, one for medium dose and one for high dose (all defined as above). The assessment thresholds (fracture probabilities for BMD testing) and intervention thresholds (fracture probabilities for therapeutic intervention) are then used in the same way as described for postmenopausal women and older men.
Men receiving androgen-deprivation therapy
Recommendations
The NOGG supports the recent guideline published by Brown et al. 2020 [267].
-
21.
All men starting androgen deprivation therapy (ADT) should have their fracture risk assessed using FRAX, considering ADT use as a secondary cause of osteoporosis, with BMD measured where available (strong recommendation).
-
22.
Consider referring men, with high fracture risk requiring drug treatment, to secondary care for assessment and initiation of treatment with bisphosphonates or denosumab (conditional recommendation).
-
23.
Men with FRAX probability near to, but below the intervention threshold, and patients going on to additional systemic therapies (particularly those requiring glucocorticoids), should have FRAX with BMD reassessed 12–18 months after starting ADT (conditional recommendation).
There is no evidence that skeletal metabolism in men differs fundamentally from that of women [268]. However, secondary causes of osteoporosis are common in men and amongst these hypogonadism is prominent [269]. Androgen deprivation therapy (ADT), used primarily in the treatment of older men with prostate cancer, is frequently associated with hypogonadism. Osteoporosis caused by ADT is associated with rapid loss of BMD within 6–12 months of initiation of ADT [270]; (evidence level Ic). There is a significant increase in fracture risk in men with prostate cancer in the 5 years following the initiation of ADT when compared to those not receiving ADT [271]; (evidence level Ic). Bisphosphonates and denosumab are effective drug treatments for preventing BMD loss in men with prostate cancer taking ADT, although effects on fracture risk have not been demonstrated. Exercise programmes are a less effective alternative which are insufficient in isolation [272]; (evidence level Ib).
In a systematic review and network meta-analysis all evaluated treatments for ADT-induced bone loss, which included bisphosphonates and selective oestrogen receptor modulators (SERMs), were effective in improving BMD compared to placebo. However, zoledronate generated greater improvements in BMD compared to other drug treatments at all bone density sites, except for risedronate which had better BMD improvement compared to zoledronate at the femoral neck site in one small study [273]; (evidence level IIa). A recent UK consensus statement on prostate cancer treatment-induced bone loss concluded that fracture risk should be calculated using FRAX, considering ADT use as a secondary cause of osteoporosis, and including BMD where available and practical. BMD should always be assessed where calculated fracture risk is close to the NOGG intervention threshold. Men requiring bone protection drug therapy should be further assessed with referral to secondary care if available and offered appropriate treatment to reduce fracture risk. Those with FRAX probability near to, but below the intervention threshold, and patients going on to additional systemic therapies (particularly those requiring glucocorticoids), should have FRAX with BMD repeated after 12–18 months [267]; (evidence level IIa).
Women receiving aromatase inhibitor therapy
Recommendations
-
24.
All women starting aromatase inhibitor (AI) therapy should have their fracture risk assessed using FRAX, considering AI use as a secondary cause of osteoporosis, including BMD measurement where practical (strong recommendation).
-
25.
Women with high fracture risk should be commenced on drug treatment to prevent
osteoporosis and fracture, with bisphosphonates or denosumab (strong recommendation).
-
26.
Women with a FRAX probability near to, but below the intervention threshold, and patients going on to additional systemic therapies (particularly those requiring glucocorticoids), should have FRAX with BMD reassessed 12–24 months after starting AI therapy (conditional recommendation).
-
27.
If adjuvant high-dose bisphosphonate therapy is used as part of breast cancer management, consider assessing fracture risk at the end of this bisphosphonate therapy, particularly if AI therapy continues (conditional recommendation).
The use of aromatase inhibitors (AI) in postmenopausal women induces bone loss at an average rate of 1–3% per year at sites rich in trabecular bone. Bone loss is more marked in young women with treatment-induced ovarian suppression, losing an average of 7–8% per annum [274]; (evidence level IIa). In case–control studies, the incidence of fracture in women with breast cancer treated with AI is reported to be around 18–20% after 5 years of follow-up [275]. NICE guidance on the management of early breast cancer, which recognises the excess risk of osteoporosis with the use of AIs, recommends a baseline DXA scan to assess BMD at the time of initiation of AI therapy [276]; (evidence level IV). International Consensus Position Statements suggest that fracture risk should be assessed, although the consideration of AI use as a secondary cause of osteoporosis in FRAX, may not adequately estimate fracture risk [275, 277]; (evidence level IIa) with drug treatment to prevent bone loss and fractures recommended in those with a T-score of less than − 2, or less than − 1.5 with 1 additional risk factor, or in those with 2 or more risk factors (without BMD). Drug treatment should be bisphosphonate (oral or parenteral) or denosumab, used in the doses for postmenopausal osteoporosis. Denosumab and zoledronate both lead to significant gains in BMD at the spine and hip in postmenopausal women with breast cancer receiving AI, and both denosumab and risedronate have been shown to reduce fracture risk [278]; (evidence level Ia).
Management of symptomatic osteoporotic vertebral fractures
Recommendations
-
1.
Administer analgesia orally rather than parenterally whenever possible. Pain should be regularly reviewed, and analgesia titrated up or down according to pain intensity and side effects, with the use of the weakest effective agent for the shortest possible time (strong recommendation).
-
2.
Avoid the use of non-steroidal anti-inflammatory drugs (NSAIDs) in older people, but, if used, co-prescribe a proton-pump inhibitor, and monitor for gastro-intestinal, renal and cardiovascular side-effects (strong recommendation).
-
3.
Prescribe appropriate laxative therapy, such as the combination of a stool softener and a stimulant laxative, whenever opioid therapy is used in older people (strong recommendation).
-
4.
It is recommended that exercise programmes following vertebral fracture include progressive muscle-strengthening activity, including back extensor muscle strengthening and/or endurance exercise (strong recommendation).
-
5.
When a patient is in pain it may be advisable to initially perform an exercise for back extensors in an unloaded position (conditional recommendation).
-
6.
Provide clear and prompt guidance on how to adapt movements involved in day-to-day living, including how exercises can help with posture and pain, to patients with painful vertebral fractures (strong recommendation).
-
7.
Ensure prompt secondary fracture prevention is started following a fracture, with follow-up through fracture liaison services for all postmenopausal women, and men aged 50 years and older, with a newly diagnosed vertebral fracture (strong recommendation).
Vertebral fractures can cause acute and chronic pain, height loss, spinal deformity and altered body shape, functional impairment, and reduced health-related quality of life [15]; (evidence level Ia). Analgesia for acute pain is important to allow restoration of function and mobility but must be used safely [279,280,281]; (evidence level IIa). In patients admitted to hospital, salmon calcitonin given for up to 4 weeks (50–100 IU daily given subcutaneously or intramuscularly), has been shown to be an effective adjunctive analgesic for pain, experienced at rest or when walking, associated with acute (within 10 days of) vertebral fracture [282]; (evidence level IIa). However, side-effects (mainly flushing and gastro-intestinal disturbance) are common. Of note, long-term use may be associated with an increased risk of cancer [283]. There is no evidence that salmon calcitonin is an effective treatment for chronic pain associated with vertebral fractures [282]; (evidence level Ia). Of note, in the SPC, calcitonin is indicated for the prevention of acute bone loss due to sudden immobilisation such as in patients with recent osteoporotic fractures, rather than for the management of pain. A single, small, randomised double-blind, controlled trial found 30 mg intravenous pamidronate, given within 21 days of acute vertebral fracture, to be more effective than placebo in reducing pain [284]; (evidence level IIb). Of note in the SPC, pamidronate is indicated for the treatment of conditions associated with increased osteoclast activity, rather than for the management of pain. Physiotherapist supervised exercise following vertebral fracture improves pain and physical performance [285]; (evidence level Ib). In the presence of pain, it may be advisable to initially perform an exercise for back extensors in an unloaded position, such as supine [286]; (evidence level Ia).
Combining exercise with physiotherapy-delivered education and guidance can reduce fear of falling and improve psychological symptoms associated with vertebral fractures [162, 287]; (evidence level Ia). For patients with painful vertebral fractures, there is low-quality evidence suggesting that spinal bracing using soft or rigid external orthoses for 2 h a day over 6 months may improve pain and trunk muscle strength [286]. There is currently no evidence that bracing with soft or rigid external orthoses improves fracture healing [288]. Hence, routine use of bracing for the treatment of acute or subacute vertebral fractures cannot be recommended (evidence level Ia). The current evidence does not support the routine use of percutaneous vertebroplasty or balloon kyphoplasty for the treatment of painful osteoporotic vertebral fractures, as these procedures do not show consistent patient benefit [286, 289]; (evidence level Ia). In older women with vertebral fractures and chronic back pain stable for 6 months or more, a small randomised control has shown electrical nerve stimulation, administered as inferential therapy or horizontal therapy 5 days a week for 2 weeks, can improve pain over 14 weeks [290]; (evidence level IIb). Patients with a recent vertebral fracture have a high imminent risk of further fragility fracture [52]; (evidence level IIb). If a vertebral fracture is associated with impending or existing neurological deficits, urgent referral to spinal surgical services is indicated.
Models of care for fracture prevention
Recommendations
-
1.
Multidisciplinary, coordinator-based FLS are recommended to systematically identify men and women with fragility fractures, facilitating timely assessment of fracture and falls risk, and where appropriate, tests to exclude secondary causes of osteoporosis, radiological investigation including BMD testing, and initiation of pharmacological and non-pharmacological interventions to reduce risk of falls and fractures (strong recommendation).
-
2.
FLSs should include embedded local audit systems supported by a clinical fracture database to enable monitoring of care provided to fracture patients (e.g., Royal College of Physicians FLS-Database); (strong recommendation).
-
3.
FLSs should employ a range of case-finding strategies to identify all inpatients and outpatients with fragility fractures (strong recommendation).
-
4.
Diagnostic imaging services should routinely evaluate the spine in all imaging of postmenopausal women, and men aged ≥ 50 years, in which the spine is visualised, and report vertebral fractures using standardised methods (strong recommendation).
-
5.
Patients recommended drug treatment for osteoporosis should be offered tailored information about osteoporosis and its treatments and further medication reviews to support adherence and to discuss alternative treatments if unacceptable adverse events arise or adherence is difficult (strong recommendation).
-
6.
Primary care clinicians should always have in mind the possibility of vertebral fracture in postmenopausal women and men age ≥ 50 years who present with acute onset back pain, especially thoracic pain if they have risk factors for osteoporosis (strong recommendation).
FLS models of care
Collaboration between primary care clinicians, secondary care physicians, orthopaedic surgeons, radiologists, and pharmacists and between the medical and non-medical disciplines concerned, should underpin secondary fracture prevention programmes. Fracture Liaison Service (FLS) programmes reduce re-fracture rates and improve survival [291, 292] (evidence levels Ia and IIb). The Department of Health and NHS RightCare both state that FLS should be provided for all patients sustaining a fragility fracture [293, 294], which aligns with the International Osteoporosis Foundation’s global Capture the Fracture® programme [295] and the Royal Osteoporosis Society (ROS) FLS Clinical Standards [296].
FLS should provide fully coordinated, intensive models of care for secondary fracture prevention. FLS models which provide identification, assessment and treatment initiation, or a treatment recommendation to primary care, are more clinically effective and cost-effective in improving patient outcomes than approaches that provide identification and/or patient alerts, and/or patient education only [297]; (evidence level Ia). The required approach is a FLS in which identification, assessment and osteoporosis treatment are all conducted within an integrated electronic health care network, overseen by a coordinator and utilizing a dedicated database measuring performance [295, 297,298,299]; (evidence level Ia). FLS that initiate pharmacological treatment, rather than making a treatment recommendation for primary care initiation, have higher rates of treatment initiation [298]; (evidence level Ia). FLS should also initiate appropriate non-pharmacological interventions and communicate ongoing care effectively with primary care practitioners [296]. FLS should provide a coordinated programme with an integrated approach for falls and fracture prevention; all individuals with a fracture should be fully assessed for falls risk and appropriate interventions to reduce falls should be undertaken [300]. As the risk of re-fracture is highest immediately after a fragility fracture, secondary fracture prevention assessment and intervention should be initiated as soon as possible, and no later than 16 weeks post-fracture, as recommended by the Royal Osteoporosis Society [52, 296].
FLS patient identification
FLSs need to employ a range of case-finding strategies, to identify both inpatients and outpatients with fragility fractures, and people with vertebral fractures who are often undiagnosed. Reasons for non-identification of vertebral fractures include the absence of a fall as a trigger for investigation, absence of symptoms, or attribution of symptoms to other causes. Furthermore, in patients who do have spinal imaging, the use of ambiguous non-standardised terminology in imaging reports, and failure to routinely evaluate the vertebrae captured in imaging of other body systems can both contribute to the non-identification of vertebral fractures. The Royal Osteoporosis Society recommend that radiology services should establish local processes to ensure that the spine is routinely evaluated for the presence of vertebral fracture in all available imaging and that reports identifying vertebral fractures should be standardised, using the words ‘vertebral fracture’, are actionable, and indicate future management [301]; (evidence level IV).
Primary care plays an important role in case finding for osteoporotic fractures, particularly vertebral fractures as acute onset back pain, especially thoracic pain, is a common presenting complaint. Targeted use of spinal imaging can help increase case identification, appropriate symptom management, and prompt secondary fracture prevention.
Providing patient information and adherence support
Patients identified by any clinical service, to be in need of further intervention, should be offered an explanation of osteoporosis, the causes, consequences and how it can be managed with pharmacological and non-pharmacological interventions. When discussing pharmacological treatment, an explanation should be offered for why drug treatment is recommended, the aims and benefits, common and/or severe side effects, the practicalities of taking the medicine, and for how long it should be taken [302]; (evidence level IV). The use of decision aids in osteoporosis to support communication of medicine risk–benefit has been shown to improve shared decision making, reduce decisional conflict and improve the accuracy of patient-perceived fracture risk [303]; (evidence level Ib). Information should be tailored to the needs of the patient to make it accessible and understandable, including the provision of written information [304].
To promote treatment adherence, healthcare professionals should elicit and address any beliefs and concerns associated with reduced adherence and establish realistic treatment expectations with the patient [302, 304]. No one type of intervention has been demonstrated to enhance medicines adherence in osteoporosis care, but multi-component models with active patient engagement have the most positive effects [305, 306]; (evidence level Ia). FLS models with a greater number of patient interactions have demonstrated greater clinical effectiveness [299]; (evidence level Ia). The NOGG supports the Royal Osteoporosis Society recommendation to follow-up within 16 weeks and 52 weeks post-fracture, to review the use of medications that increase the risk of falls and/or fracture, to ensure co-prescription of calcium and vitamin D with bone protective interventions where indicated, to review adverse effects and monitor adherence to therapy [296].
Recommendations for training
Recommendations
The following are recommended:
-
1.
Training in personalised care, including shared decision making, is provided within all higher professional training curricula in relevant medicine and surgical specialities (strong recommendation).
-
2.
Training in osteoporosis and metabolic bone diseases is a clearly articulated component of each of the relevant medical and surgical specialities higher professional training curricula set out by the applicable medical and surgical Royal Colleges (strong recommendation).
-
3.
Primary care physicians have sufficient training in this area with efficient access to up-to-date evidence-based resources and guidelines, and continual professional development (CPD) opportunities to maintain and refine knowledge (strong recommendation).
-
4.
The management of osteoporosis is a component of training in all relevant allied health disciplines (strong recommendation).
-
5.
Training should be provided to Fracture Liaison Service personnel to achieve high-quality DXA performance and reporting (strong recommendation).
-
6.
Quality improvement training should be provided to healthcare personnel responsible for the delivery of Fracture Liaison and/or Osteoporosis Services (strong recommendation).
The management of osteoporosis and fragility fracture risk is not subserved by any one specialty. The relevant medical and surgical specialties include general practice, rheumatology, orthopaedic surgery, endocrinology, metabolic medicine, geriatric medicine, and obstetrics and gynaecology. Furthermore, the care of patients with osteoporosis is the responsibility of multiple healthcare professionals, including nurses, physiotherapists, occupational therapists, pharmacists and DXA operators. The multi-disciplinary nature of osteoporosis care offers opportunities for cross-speciality training. It is recognised that primary care is pivotal to the identification of the population at risk of fragility fractures as well as to the long-term management of patients with osteoporosis. It is important that primary care physicians have sufficient training in this area, with access to resources such as updated guidelines and online learning modules to refresh their knowledge.
Common to all healthcare roles is a need to provide personalised patient-centred care, a key commitment outlined by the NHS to be achieved by 2023/24. Personalised care is a partnership approach that helps people make informed decisions and choices about their health and wellbeing, working alongside clinical information (Personalised Care Institute 2020). There is significant variability in the access to and quality of DXA services for established FLS worldwide. Despite two decades of training initiatives in osteoporosis densitometry, many centres are falling short of the standards of the IOF-ISCD Osteoporosis Essentials criteria [307].
Improving the quality of osteoporosis and fracture liaison services is about making health care delivery safe, effective, patient-centred, timely, efficient, and equitable. Quality improvement involves the use of a systematic and coordinated approach to solving a problem using specific methods and tools with the aim of bringing about a measurable improvement within a health care setting [308] and can be aided by the use of appropriate Toolkits (e.g. the Royal Osteoporosis Society Fracture Liaison Service Implementation Toolkit).
Examples of appropriate training
-
i.
Training in Personalised Care. The Personalised Care Institute is a virtual organisation, accountable for setting the standards for evidence-based training in personalised care in England. The Personalised Care Institute Curriculum sets out the standards for training programmes to become accredited with the Personalised Care Institute. The Personalised Care Institute provides eLearning modules for example on Shared Decision Making. The curriculum is designed for health care personnel within primary and secondary care and community teams https://www.personalisedcareinstitute.org.uk.
-
ii.
Training in Osteoporosis Management. The Royal Osteoporosis Society Fracture Prevention Practitioner Training is accredited for CPD by RCGP, RCP and RCN. The online training includes five foundation modules and then three advanced modules https://theros.org.uk/healthcare-professionals/courses-and-cpd/fracture-prevention-practitioner-training/. The Royal College of General Practice also provides a short e-Learning module on the diagnosis and management of osteoporosis https://elearning.rcgp.org.uk/course/info.php?id=233
-
iii.
Training in Musculoskeletal Pain Management. The Health Education England e-Learning for Healthcare Pain Management programme includes training on musculoskeletal pain which encompasses the assessment and management of osteoporotic vertebral fractures https://www.e-lfh.org.uk/programmes/pain-management/.
-
iv.
Training in DXA conduct and reporting. The Royal Osteoporosis Society run a Bone Densitometry Foundation Course. This online course provides a foundation in osteoporosis and DXA (https://theros.org.uk/healthcare-professionals/courses-and-cpd/bone-densitometry-foundation-course/).
Recommendations for commissioners of healthcare
In 2017, the National Falls Prevention Coordination Group with Public Health England (PHE) produced a falls and fracture consensus statement and resource pack with the aim of reducing falls and fracture risk and improving the management of fractures, including secondary prevention (https://www.gov.uk/government/publications/falls-and-fractures-consensus-statement). The guidance is aimed at local commissioning and strategic leads in England with a remit for falls, bone health and healthy ageing. Following this, NHS RightCare, working with PHE and the Royal Osteoporosis Society (ROS), developed a Falls and Fragility Fractures Pathway (https://www.england.nhs.uk/rightcare/products/pathways/falls-and-fragility-fractures-pathway/) which defines three priorities that commissioners responsible for falls and fragility fractures should optimise as a priority: (i) falls prevention, (ii) detecting and managing osteoporosis, and (iii) optimal support after a fragility fracture. The ROS has developed an online Fracture Liaison Service Implementation Toolkit (https://theros.org.uk/healthcare-professionals/fracture-liaison-services/implementation-toolkit/) designed to enable FLS Commissioning. In England, the move to Integrated Care Systems (ICS) provides an opportunity to embed enhanced pathways of care for patients at risk of fragility fracture, including imminent fracture risk [309], as part of routine service delivery, for example enabling direct referrals between different secondary care services to streamline patient care pathways.
Where healthcare funding is not delivered through a commissioning structure the recommendations below apply to bodies providing healthcare funding and to local health boards. Thus, in Wales, these recommendations apply to the Welsh Government and to local health boards (that are funded directly from the Welsh Government) when setting their Integrated Medium-Term Plans (IMTPs). In Northern Ireland, health and social care are integrated and are the responsibility of the Department of Health. Health services are commissioned by the Health and Social Care Board (HSCB) through local commissioning groups from the five Health and Social Care Trusts. Thus, in Northern Ireland, these recommendations apply to the HSCB and to the five local commissioning groups.
Recommendations
Based upon the evidence presented in this guideline, the NOGG makes the following recommendations to service leaders and/or commissioners of healthcare:
-
1.
Should recognise that fractures due to osteoporosis are a significant and growing public health issue with consequent high health and social care costs and ensure that fragility fractures are addressed explicitly in their local healthcare programmes (strong recommendation).
-
2.
Should ensure that local healthcare programmes address approaches to reduce the prevalence of avoidable risk factors for osteoporosis and fractures related to falls and poor bone health and, in so doing, makes explicit the roles of both the NHS and other agencies (strong recommendation).
-
3.
Should ensure electronic patient health record systems have FRAX, and the link to the NOGG website, integrated to aid identification and treatment of those at risk of fragility fracture, and that electronic patient health record systems enable clear, and where possible automated, electronic communication between FLS and primary care teams (strong recommendation).
-
4.
Should put arrangements in place so that those at risk of osteoporotic fractures have the opportunity to receive appropriate investigation (e.g., fracture risk assessment, falls risk assessment, bone density measurement), lifestyle advice (e.g., about diet, exercise, and smoking), and bone protective drug therapy (NICE Quality Standards 149, 2017). The latter includes the availability of parenteral drug therapies in 70 and community healthcare settings (strong recommendation).
-
5.
Should ensure that accurate, up-to-date consistent information about pharmacological drug interventions is widely available to postmenopausal women, and men aged ≥ 50 years, their healthcare advocates, and professional advisers, so that patients can make informed decisions about treatment and treatment adherence (strong recommendation).
-
6.
Integrated Care Systems (ICS) should specifically address the burden of fragility fractures on the local economy and ensure that Fracture Liaison Services are available for all patients who sustain a fragility fracture (strong recommendation).
-
7.
ICS should bring together local specialists, generalists and other stakeholders, including patient representatives, to agree local treatment practices and referral pathways for the management of osteoporosis and prevention of fragility fractures. It is often helpful to identify a lead clinician in both primary and secondary care. The recommendations of this group should take account of local resources and relevant cost-effectiveness data. Local guidelines should be consistent with the evidence presented in this document. Once local guidelines have been agreed, they should be widely disseminated to relevant professionals and potential patients, and the necessary service changes made to allow the guidelines to be implemented. Implementation should be audited and appropriate changes in practice should be instituted where standards are not met with appropriate monitoring of compliance to guidelines thereafter (strong recommendation).
Review criteria for audit and quality improvement
Quality standards for osteoporosis
-
1.
Four quality standards for osteoporosis were produced by the National Institute for Health and Care Excellence (NICE) in 2017 (QS149) (https://www.nice.org.uk/guidance/qs149).
-
2.
Seven quality standards for osteoporosis and the prevention of fragility fractures were produced by the Royal Osteoporosis Society in 2017 (https://theros.org.uk/media/0dillsrh/ros-op-standards-november-2017.pdf)
Primary care
-
3.
Documentation of the proportion of postmenopausal women, and men aged ≥ 50 years, registered with a general practice:
-
a)
With a fracture code, who have been assessed to determine whether their fracture was a fragility (low-trauma) fracture
-
b)
With one or more risk factors for fragility fracture, who receive formal fracture risk assessment.
-
c)
With a prior fragility fracture, who have had a DXA scan with the result recorded.
-
d)
Calculated to be high or very high risk by FRAX assessment, who have been offered drug treatment.
-
e)
With an incident hip fracture, those who receive pharmacological drug therapy for osteoporosis within 16 weeks of their fracture.
-
f)
Who are prescribed pharmacological drug therapy for osteoporosis and who have had confirmed adherence to osteoporosis therapy within the last 12 months.
-
g)
Who are prescribed pharmacological drug therapy for osteoporosis and have had a 5-year and 10-year review.
-
h)
Who are prescribed denosumab, who have received timely (within 4 weeks of due date) follow-up injection.
-
i)
Who are on oral glucocorticoids for ≥ 3 months who have had a fracture risk assessment.
-
j)
With documented discussion of fracture risk assessment and a treatment decision.
-
a)
Fracture liaison services
-
4.
The Royal Osteoporosis Society (ROS) published in 2019 six key standards for FLS with a corresponding timeline for the achievement of these six steps, with examples of audit and evidence [296].
-
5.
The Royal College of Physicians Fracture Liaison Service (FLS) Database National Audit (https://www.rcplondon.ac.uk/projects/fracture-liaison-service-database-fls-db) is commissioned by the Healthcare Quality Improvement Partnership (HQIP) as part of the Falls and Fragility Fracture Audit Programme. The FLS-DB is included in the HQIP listing for national audits that must be reported in each English hospital trust’s Quality Account and is required by the Welsh Government for all Health Boards in Wales. These form part of the National Clinical Audit Patient Outcomes Programme. All FLS sites that treat fractures are eligible to participate. The FLS-DB sets out 11 Key Performance Indicators (KPIs) which are designed to measure performance against technology assessments, guidance on osteoporosis and clinical standards for FLSs from the NICE, the ROS and NOGG.
-
6.
The International Osteoporosis Foundation (IOF) Capture the Fracture Best Practice Framework outlines 13 standards for FLS delivery with criteria and targets specified for bronze, silver or gold levels of achievement (https://www.capturethefracture.org/best-practice-framework).
DXA reporting
-
6.
The ROS published in 2019 six quality standards for DXA reporting with a corresponding audit template [40].
Summary of main recommendations
This guideline summary addresses the assessment, diagnosis and current treatments for osteoporosis, including recommendations to prevent fragility fractures. It applies to postmenopausal women, and to men aged 50 years or older.
Concerning the assessment of fracture risk in postmenopausal women, and men aged ≥ 50
-
1.
Conduct a FRAX assessment in people with a clinical risk factor for fragility fracture.
-
2.
Measure BMD in people with intermediate fracture risk by FRAX (amber) to refine the estimate of 10-year risk.
-
3.
Measure BMD in people with high and very high fracture risk by FRAX (red) to guide drug choice and provide a baseline for BMD monitoring.
-
4.
Consider imaging to look for a vertebral fracture in people with acute onset back pain who have risk factors for osteoporosis, and/or in people with a history of ≥ 4 cm height loss, kyphosis, recent or current long-term oral glucocorticoid therapy, or a BMD T-score ≤ − 2.5.
-
5.
Assess falls risk in patients with osteoporosis and/or fragility fractures and offer those at risk an exercise programme to improve balance and muscle strength.
Regarding drug treatment to prevent fractures in postmenopausal women, and men aged ≥ 50
-
6.
Offer drug treatment to people at high and very high risk of fracture.
-
7.
If BMD measurement is not practical (e.g., due to frailty), use the online NOGG intervention thresholds based on FRAX, to guide treatment decisions.
-
8.
Consider, particularly in older people, drug treatment in those with a prior and/or recent fragility fracture.
When selecting drug treatments to prevent fractures in postmenopausal women, and men aged ≥ 50
-
9.
Consider the level of fracture risk, any additional clinical risk factors, patient choice, and the cost-effectiveness of treatment, when deciding on a particular drug treatment.
-
10.
Start treatment promptly following a fragility fracture, because the risk of re-fracture is highest immediately after a fracture, and risk remains elevated.
-
11.
Consider referral of very high-risk patients to an osteoporosis specialist in secondary care, for assessment and consideration of parenteral treatment (some may need first-line anabolic drug treatment, especially if multiple vertebral fractures). Indications for specialist referral include the presence of important risk factors, including a recent vertebral fracture (within the last 2 years), ≥ 2 vertebral fractures (whenever they have occurred), BMD T-score ≤ − 3.5, treatment with high dose glucocorticoids (≥ 7.5 mg/day of prednisolone or equivalent over 3 months); the presence of multiple clinical risk factors, particularly with a recent fragility fracture indicating high imminent risk of re-fracture; or other indicators of very high fracture risk.
-
12.
In other patients for whom treatment is indicated, offer antiresorptive therapy with oral bisphosphonates (alendronate or risedronate) or intravenous zoledronate.
-
13.
Consider alternative treatment options if first-line bisphosphonates are unsuitable or not tolerated; denosumab, ibandronate, hormone replacement therapy, raloxifene or strontium ranelate.
-
14.
Following treatment with teriparatide or romosozumab, start alendronate, zoledronate or denosumab without delay.
When postmenopausal women, and men aged ≥ 50, have started drug treatment
-
15.
Regularly review patients' tolerance of, and adherence to, oral drug treatments.
-
16.
Remember long-term treatment is often required, because osteoporosis is a long-term condition for which there is currently no cure.
-
17.
Plan to prescribe oral bisphosphonates for at least 5 years, or intravenous bisphosphonates for at least 3 years and then re-assess fracture risk. Longer durations of treatment will be needed in those who are older (age ≥ 70 years), have had a hip or vertebral fracture, are on high-dose oral glucocorticoids (≥ 7.5 mg/day of prednisolone or equivalent over 3 months), or have a further fragility fracture during osteoporosis treatment. In lower-risk patients, a temporary treatment pause of 18 to 36 months can be considered after 5 years’ oral bisphosphonate or 3 years’ intravenous bisphosphonate (see clinical flowcharts on p.39 and p.40).
-
18.
Before starting denosumab, ensure a long-term personalised osteoporosis management plan is in place.
-
19.
Do not stop denosumab treatment without a plan for subsequent anti-resorptive therapy, where renal function permits.
-
20.
Repeat fracture risk assessment after any new fracture, regardless of when this occurs.
-
21.
Reassess fracture risk 18 months to 3 years after pausing drug treatment.
When postmenopausal women, and men aged ≥ 50, are treated with oral glucocorticoids
-
22.
If starting ≥ 7.5 mg/day prednisolone or equivalent for the next 3 months, start bone protective treatment at the same time (without waiting for a DXA scan, which can follow later).
-
23.
Offer antiresorptive therapy with oral bisphosphonates (alendronate or risedronate) or intravenous zoledronate, and in those at very high risk of vertebral fracture refer for consideration of anabolic treatment.
-
24.
Consider denosumab as an alternative treatment option.
When advising on lifestyle and dietary measures
-
25.
Recommend a healthy, balanced diet, moderation of alcohol consumption and avoidance of smoking.
-
26.
Ensure a sufficient dietary calcium and vitamin D intake and supplement these as necessary.
-
27.
Encourage a combination of regular weight-bearing and muscle-strengthening exercise.
Regarding fracture prevention services
-
28.
Patients who sustain a fragility fracture should have access to a multidisciplinary, coordinator-based Fracture Liaison Service (FLS) which enables timely fracture and falls risk assessment, investigation, treatment, and monitoring.
-
29.
Ensure that diagnostic imaging services routinely evaluate the spine in all imaging of postmenopausal women, and men aged ≥ 50 years, in which the spine is visualised, and report vertebral fractures using standardised methods.
When a postmenopausal woman, or a man aged ≥ 50, has a symptomatic osteoporotic vertebral fracture
-
30.
Consider referral to an exercise programme that provides progressive muscle-strengthening activity, including back extensor muscle strengthening and/or endurance exercise.
-
31.
Investigate for underlying causes of fragility fracture.
-
32.
Start treatment promptly to reduce the risk of further fractures.
The evidence presented in this guideline underpins a further series of recommendations made for leaders and commissioners of healthcare services, as well as criteria for audit and quality improvement in primary and secondary care settings.
Appendix 1
List of stakeholders
Association for Clinical Biochemistry and Laboratory Medicine.
Bone Research Society.
British Geriatrics Society.
British Orthopaedic Association.
British Orthopaedic Research Society.
British Menopause Society.
British Society for Rheumatology.
European Calcified Tissues Society.
European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases
International Osteoporosis Foundation.
Osteoporosis 2000.
Osteoporosis Dorset.
Primary Care Rheumatology and Musculoskeletal Medicine Society.
Royal College of Physicians.
Royal Osteoporosis Society.
Royal Pharmaceutical Society.
Society for Endocrinology.
The Nutrition Society.
External reviewers
1. Prof. William Leslie, University of Manitoba, Canada.
2. Dr. Nicola Peel, Sheffield Teaching Hospitals NHS Foundation Trust, UK.
3. Dr. Stephen Tuck, South Tees Hospitals NHS Foundation Trust, UK.
Appendix 2. Grading of evidence
Levels of evidence for studies of intervention
- Ia :
-
from systematic review and meta-analysis of randomised controlled trials (RCTs)
- Ib :
-
individual RCT(s) (with narrow confidence intervals).
- Ila :
-
systematic review of at least one non-randomised controlled trial or well-designed cohort study.
- IIb :
-
individual cohort study or low-quality RCTs.
- IIIa :
-
systematic review of at least one case-controlled study.
- IIIb :
-
individual case–control study.
- IV :
-
expert committee reports or opinions and/or clinical experience of authorities, case series (and poor-quality cohort and case–control studies).
Levels of evidence for the validity of candidate risk factors
- Ia :
-
Systematic reviews or meta-analysis of level I studies with a high degree of homogeneity.
- Ib :
-
Systematic reviews or meta-analysis with moderate or poor homogeneity.
- Ic :
-
Level I studies (with appropriate populations and internal controls).
- IIa :
-
Systematic reviews or meta-analysis of level II studies.
- IIb :
-
Level II studies (inappropriate population or lacking an internal control).
- IIIa :
-
Systematic reviews or meta-analysis of level III studies.
- IIIb :
-
Case-control studies
- IV :
-
Evidence from expert committees without explicit critical scientific analysis or that based on physiology, basic research or first principles.
Of note, FRAX risk factors are all grade A or B according to evidence for reversibility of risk [64].
Grading of recommendations
Recommendations follow the Grading of Recommendations Assessment, Development, and Evaluation GRADE binary classification of recommendations as either strong or conditional (also known as discretionary or qualified recommendations) [310]. Recommendations have been made after the assessment of [311]:
-
1.
The balance between desirable and undesirable effects -The larger the difference between the desirable and undesirable effects, the more likely a strong recommendation is warranted.
-
2.
The quality of evidence—The higher the quality of evidence, the more likely a strong recommendation is warranted.
-
3.
Values and preferences—The more variability/ uncertainty in values and preferences the more likely a conditional recommendation is warranted.
-
4.
Costs (resource allocation)—The higher the costs of an intervention (i.e., the more resources consumed) the more likely a conditional recommendation is warranted.
For example, a strong recommendation applies where the clinician considers that most people ought to receive the intervention, or where adherence to the recommendation could be used as a performance or quality indicator and that deviation from this recommendation would prompt documentation of a clinician’s rationale. NICE suggests using ‘offer’ (or similar action wording such as ‘measure’, ‘advise’, ‘commission’ or ‘refer’) when describing a strong recommendation [312].
A conditional recommendation applies where the clinician examines the evidence and prepares to discuss this with the patient together with the patient’s values and preferences, or where documentation of the discussion of the pros and cons of an intervention is the indicator of quality, rather than the course of action itself. NICE suggests using wording such as ‘consider’ when describing conditional recommendations. Where insufficient evidence is available or the evidence available is equivocal, recommendations are not made.
Appendix 3. AMSTAR2 grading of systematic reviews and meta-analyses
The quality of systematic reviews and meta-analyses used in the formulation of recommendations was assessed using AMSTAR2 (https://amstar.ca/Amstar-2.php).
Topic | Reference | Type of study | AMSTAR2 grading | Reference |
|---|---|---|---|---|
Fracture risk assessment and case finding | Bai et al. 2020 | MA | Low | [60] |
Gausden et al. 2017 | SR | Medium | [100] | |
Johannesdottir et al. 2018 | SR | Low | [42] | |
Kanis et al. 2016 | SR | Medium | [79] | |
Marshall et al. 1996 | MA | Critically Low | [29] | |
Merlijn et al. 2019 | SR & MA | Critically Low | [107] | |
Mortensen et al. 2020 | SR & MA | Medium | [66] | |
Singh-Ospina et al. 2017 | SR & MA | Low | [73] | |
Vilaca et al. 2020 | SR & MA | Low | [59] | |
Zhang et al. 2020 | SR & MA | Low | [109] | |
Intervention thresholds and management strategy | Kanis et al. 2016 | SR | Medium | [79] |
Non-pharmacological management of osteoporosis | Babatunde et al. 2020 | SR & MA | Medium | [160] |
El-Khoury et al. 2013 | SR & MA | Medium | [166] | |
Darling et al. 2019 | SR & MA | Medium | [144] | |
Fabiani et al. 2019 | SR & MA | Medium | [141] | |
Gillespie et al. 2012 | SR & MA | High | [170] | |
Groenendijk et al. 2019 | SR & MA | Medium | [143] | |
Iguacel et al. 2018 | SR & MA | High | [146] | |
Howe et al. 2011 | SR & MA | High | [158] | |
Jepsen et al. 2017 | SR & MA | Medium | [171] | |
Kahwati et al. 2018 | SR & MA | Medium | [155] | |
Kelley et al. 2000 | SR & MA | Medium | [161] | |
Kemmler et al. 2020 | SR & MA | Low | [159] | |
Kunutsor et al. 2018 | SR & MA | Medium | [163] | |
Min et al. 2017 | SR & MA | Low | [174] | |
Shen et al. 2015 | SR & MA | Medium | [173] | |
Sherrington et al. 2017 | SR & MA | Low | [169] | |
Sherrington et al. 2019 | SR & MA | High | [167] | |
Yao et al. 2019 | SR & MA | Medium | [151] | |
Zhao et al. 2019 | SR & MA | Low | [168] | |
Pharmacological treatment options | Diez-Perez et al. 2019 | SR & MA | Medium | [228] |
Gartlehner et al. 2017 | SR & MA | Medium | [215] | |
Nayak et al. 2017 | SR & MA | Low | [313] | |
Poon et al. 2018 | SR & MA | Low | [273] | |
Simpson et al. 2020 | SR & MA | Medium | [229] | |
Zeng et al. 2019 | SR & MA | Medium | [314] | |
Strategies for management of osteoporosis and fracture risk | Deng et al. 2020 | SR & MA | Low | [266] |
Dennison et al. 2019 | SR | Medium | [204] | |
Gedmintas et al. 2013 | SR & MA | Medium | [259] | |
Khan et al. 2015 | SR | Medium | [252] | |
Miyashita et al. 2020 | SR & MA | Low | [278] | |
Nayak et al. 2019 | SR & MA | High | [241] | |
Tsourdi et al. 2020 | SR | Medium | [202] | |
Wang et al. 2018 | SR & MA | Critically Low | [315] | |
Yanbeiy et al. 2019 | SR & MA | Low | [316] | |
Management of symptomatic osteoporotic vertebral fractures | Al-Sari et al. 2016 | SR & MA | Low | [15] |
British Geriatric Society 2013 | SR | Medium | [281] | |
Buchbinder et al. 2018 | SR & MA | High | [289] | |
Ebeling et al. 2019 | SR & MA | Critically Low | [286] | |
Gibbs et al. 2019 | SR | Medium | [285] | |
Hofler et al. 2020 | SR | Low | [288] | |
Knopp-Sihota et al. 2012 | SR & MA | Medium | [282] | |
Svensson et al. 2017 | SR | Low | [287] | |
Models of care for fracture prevention | Ganda et al. 2013 | SR & MA | Critically Low | [297] |
Ganda et al. 2019 | SR & MA | Low | [298] | |
Martin et al. 2020 | SR & MA | Medium | [306] | |
Paskins et al. 2020 | SR | Medium | [303] | |
Wu et al. 2018 | SR | Critically Low | [292] | |
Wu et al. 2018 | SR & MA | Low | [299] |
SR systematic review; MA meta-analysis.
Change history
19 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11657-022-01115-8
References
Compston J, Cooper A, Cooper C, Francis R, Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P, Wilkins M (2009) Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas 62:105–108
Compston J, Bowring C, Cooper A et al (2013) Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas 75:392–396
Compston J, Cooper A, Cooper C et al (2017) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 12:43
Harvey NC, McCloskey E, Kanis JA, Compston J, Cooper C (2018) Cost-effective but clinically inappropriate: new NICE intervention thresholds in osteoporosis (Technology Appraisal 464). Osteoporos Int 29:1511–1513
Söreskog E, Lindberg I, Kanis JA, Åkesson KE, Willems D, Lorentzon M, Ström O, Berling P, Borgström F (2021) Cost-effectiveness of romosozumab for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture in Sweden. Osteoporos Int 32:585–594
Shea BJ, Reeves BC, Wells G, et al. (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed) 358:j4008
Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
van Staa TP, Dennison EM, Leufkens HG, Cooper C (2001) Epidemiology of fractures in England and Wales. Bone 29:517–522
Borgström F, Karlsson L, Ortsäter G et al (2020) Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos 15:59
Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA, Hochberg MC, Vogt MT, Orwoll ES, for the Study of Osteoporotic Fractures Research G (2005) Hip Fracture in Women without Osteoporosis. J Clin Endocrinol Metab 90:2787–2793
Schuit SCE, van der Klift M, Weel AEAM, de Laet CEDH, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JPTM, Pols HAP (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34:195–202
Kanis JA, Oden A, Johnell O et al (2007) The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int 18:1033–1046
Royal College of Physicians (2019) National Hip Fracture Database Annual Report. https://www.nhfd.co.uk/files/2019ReportFiles/NHFD_2019_Annual_Report_v101.pdf
NHS National Services Scotland (2019) Scottish Hip Fracture Audit. Hip Fracture Care Pathway Report 2019. https://www.shfa.scot.nhs.uk/Reports/_docs/2019-08-20-SHFA-Report.pdf
Al-Sari UA, Tobias J, Clark E (2016) Health-related quality of life in older people with osteoporotic vertebral fractures: a systematic review and meta-analysis. Osteoporos Int 27:2891–2900
Griffin XL, Parsons N, Achten J, Fernandez M, Costa ML (2015) Recovery of health-related quality of life in a United Kingdom hip fracture population. The Warwick Hip Trauma Evaluation--a prospective cohort study. Bone Joint J, 97-b:372–382
Leal J, Gray AM, Prieto-Alhambra D, Arden NK, Cooper C, Javaid MK, Judge A (2016) Impact of hip fracture on hospital care costs: a population-based study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 27:549–558
Kanis JA, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, McCloskey EV, Willers C, F B (2021) SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos. https://doi.org/10.1007/s11657-020-00871-9
Royal College of Physicians (2018) National Hip Fracture Database Annual Report. https://www.nhfd.co.uk/files/2018ReportFiles/NHFD-2018-Annual-Report-v101.pdf
Royal College of Physicians (2021) The challenge of the next decade: are hip fracture services ready? A review of data from the National Hip Fracture Database (January–December 2019). RCP, London
Hawley S, Javaid MK, Prieto-Alhambra D, Lippett J, Sheard S, Arden NK, Cooper C, Judge A (2016) Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing 45:236–242
Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR (2009) Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 301:513–521
Johnell O, Kanis JA, Odén A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jönsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone 32:468–473
Bhimjiyani A, Neuburger J, Jones T, Ben-Shlomo Y, Gregson CL (2018) The effect of social deprivation on hip fracture incidence in England has not changed over 14 years: an analysis of the English Hospital Episodes Statistics (2001–2015). Osteoporos Int 29:115–124
Bhimjiyani A, Neuburger J, Jones T, Ben-Shlomo Y, Gregson CL (2018) Inequalities in hip fracture incidence are greatest in the North of England: regional analysis of the effects of social deprivation on hip fracture incidence across England. Public Health 162:25–31
Curtis EM, van der Velde R, Moon RJ, van den Bergh JP, Geusens P, de Vries F, van Staa TP, Cooper C, Harvey NC (2016) Epidemiology of fractures in the United Kingdom 1988–2012: variation with age, sex, geography, ethnicity and socioeconomic status. Bone 87:19–26
van der Velde RY, Wyers CE, Curtis EM, Geusens PPMM, van den Bergh JPW, de Vries F, Cooper C, van Staa TP, Harvey NC (2016) Secular trends in fracture incidence in the UK between 1990 and 2012. Osteoporos Int 27:3197–3206
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312:1254–1259
Johnell O, Kanis JA, Oden A et al (2005) Predictive value of BMD for hip and other fractures. J Bone Miner Res 20:1185–1194
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936
Kanis JA, Gluer CC (2000) An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors. International Osteoporosis Foundation OsteoporosInt 11:192–202
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42:467–475
De Laet CE, Van Hout BA, Burger H, Weel AE, Hofman A, Pols HA (1998) Hip fracture prediction in elderly men and women: validation in the Rotterdam study. J Bone Miner Res 13:1587–1593
Binkley N, Adler R, Bilezikian JP (2014) Osteoporosis diagnosis in men: the T-score controversy revisited. Curr Osteoporos Rep 12:403–409
International Society for Clinical Densitometry (ISCD) (2019) Adult Official Positions of the ISCD. https://iscd.org/learn/official-positions/adult-positions/
Kanis JA, Johnell O, Oden A et al (2006) The use of multiple sites for the diagnosis of osteoporosis. Osteoporos Int 17:527–534
Leslie WD, Tsang JF, Caetano PA, Lix LM (2007) Number of osteoporotic sites and fracture risk assessment: a cohort study from the Manitoba Bone Density Program. J Bone Miner Res 22:476–483
Leslie WD, Martineau P, Bryanton M, Lix LM (2019) Which is the preferred site for bone mineral density monitoring as an indicator of treatment-related anti-fracture effect in routine clinical practice? A registry-based cohort study. Osteoporos Int 30:1445–1453
Royal Osteoporosis Society (2019) Reporting dual energy X-ray absorptiometry scans in adult fracture risk assessment: Standards for quality. https://theros.org.uk/media/xhfhyy52/ros-reporting-dxa-scans-in-adult-fracture-risk-assessment-august-2019.pdf
Adams AL, Fischer H, Kopperdahl DL et al (2018) Osteoporosis and hip fracture risk from routine computed tomography scans: the Fracture, Osteoporosis, and CT Utilization Study (FOCUS). J Bone Miner Res 33:1291–1301
Johannesdottir F, Allaire B, Bouxsein ML (2018) Fracture prediction by computed tomography and finite element analysis: current and future perspectives. Curr Osteoporos Rep 16:411–422
Pickhardt PJ, Bodeen G, Brett A, Brown JK, Binkley N (2015) Comparison of femoral neck BMD evaluation obtained using Lunar DXA and QCT with asynchronous calibration from CT colonography. J Clin Densitom 18:5–12
Cann CE, Adams JE, Brown JK, Brett AD (2014) CTXA hip–an extension of classical DXA measurements using quantitative CT. PLoS ONE 9:e91904–e91904
Ziemlewicz TJ, Maciejewski A, Binkley N, Brett AD, Brown JK, Pickhardt PJ (2016) Opportunistic quantitative CT bone mineral density measurement at the proximal femur using routine contrast-enhanced scans: direct comparison with DXA in 355 adults. J Bone Miner Res 31:1835–1840
Lenchik L, Weaver AA, Ward RJ, Boone JM, Boutin RD (2018) Opportunistic screening for osteoporosis using computed tomography: state of the art and argument for paradigm shift. Curr Rheumatol Rep 20:74
Kanis JA on behalf of the WHO Scientific Group (2007) Assessment of osteoporosis at the primary health-care level. Technical Report. WHO Collaborating Centre, University of Sheffield, UK, Sheffield 2008
Laet C, Kanis J, Oden A et al (2005) Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 16:1330–1338
Leslie WD, Schousboe JT, Morin SN, Martineau P, Lix LM, Johansson H, McCloskey EV, Harvey NC, Kanis JA (2020) Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int 31:1059–1067
Kanis J, Johnell O, Laet CD et al (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375–382
Johansson H, Odén A, McCloskey EV, Kanis JA (2014) Mild morphometric vertebral fractures predict vertebral fractures but not non-vertebral fractures. Osteoporos Int 25:235–241
Kanis JA, Johansson H, Odén A et al (2018) Characteristics of recurrent fractures. Osteoporos Int 29:1747–1757
Kanis JA, Johansson H, Harvey NC, Gudnason V, Sigurdsson G, Siggeirsdottir K, Lorentzon M, Liu E, Vandenput L, McCloskey EV (2020) Adjusting conventional FRAX estimates of fracture probability according to the recency of sentinel fractures. Osteoporos Int 31:1817–1828
Kanis JA, Johansson H, Oden A et al (2004) A family history of fracture and fracture risk: a meta-analysis. Bone 35:1029–1037
Kanis JA, Johnell O, Oden A et al (2005) Smoking and fracture risk: a meta-analysis. Osteoporos Int 16:155–162
van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 39:1383–1389
Kanis JA, Johansson H, Oden A et al (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Mineral Research 19:893–899
Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, Pols H, Tenenhouse A (2005) Alcohol intake as a risk factor for fracture. Osteoporos Int 16:737–742
Vilaca T, Schini M, Harnan S, Sutton A, Poku E, Allen IE, Cummings SR, Eastell R (2020) The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: a systematic review and meta-analysis update. Bone 137:115457
Bai J, Gao Q, Wang C, Dai J (2020) Diabetes mellitus and risk of low-energy fracture: a meta-analysis. Aging Clin Exp Res 32:2173–2186
Leslie WD, Rubin MR, Schwartz AV, Kanis JA (2012) Type 2 diabetes and bone. J Bone Miner Res 27:2231–2237
Giangregorio LM, Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA (2012) FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res 27:301–308
Johansson H, Kanis JA, Oden A, Johnell O, McCloskey E (2009) BMD, clinical risk factors and their combination for hip fracture prevention. Osteoporos Int 20:1675–1682
Kanis JA, McCloskey E, Johansson H, Oden A, Leslie WD (2012) FRAX(®) with and without bone mineral density. Calcif Tissue Int 90:1–13
McClung MR, Geusens P, Miller PD et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344:333–340
Mortensen SJ, Mohamadi A, Wright CL, Chan JJ, Weaver MJ, von Keudell A, Nazarian A (2020) Medications as a risk factor for fragility hip fractures: a systematic review and meta-analysis. Calcif Tissue Int 107:1–9
Vestergaard P, Rejnmark L, Mosekilde L (2007) Fracture risk associated with parkinsonism and anti-Parkinson drugs. Calcif Tissue Int 81:153–161
Arbouw ME, Movig KL, van Staa TP, Egberts AC, Souverein PC, de Vries F (2011) Dopaminergic drugs and the risk of hip or femur fracture: a population-based case-control study. Osteoporos Int 22:2197–2204
Lee YK, Lee EG, Kim HY, Lee Y, Lee SM, Suh DC, Yoo JI, Lee S (2020) Osteoporotic fractures of the spine, hip, and other locations after adjuvant endocrine therapy with aromatase inhibitors in breast cancer patients: a meta-analysis. J Korean Med Sci 35:e403
Myint ZW, Momo HD, Otto DE, Yan D, Wang P, Kolesar JM (2020) Evaluation of fall and fracture risk among men with prostate cancer treated with androgen receptor inhibitors: a systematic review and meta-analysis. JAMA network open 3:e2025826
Cosman F, Cauley JA, Eastell R, Boonen S, Palermo L, Reid IR, Cummings SR, Black DM (2014) Reassessment of fracture risk in women after 3 years of treatment with zoledronic acid: when is it reasonable to discontinue treatment? J Clin Endocrinol Metab 99:4546–4554
Palermo A, D’Onofrio L, Eastell R, Schwartz AV, Pozzilli P, Napoli N (2015) Oral anti-diabetic drugs and fracture risk, cut to the bone: safe or dangerous? A narrative review. Osteoporos Int 26:2073–2089
Singh-Ospina N, Maraka S, Rodriguez-Gutierrez R, Davidge-Pitts C, Nippoldt TB, Prokop LJ, Murad MH (2017) Effect of sex steroids on the bone health of transgender individuals: a systematic review and meta-analysis. J Clin Endocrinol Metab 102:3904–3913
Diez-Perez A, Adachi JD, Agnusdei D et al (2012) Treatment failure in osteoporosis. Osteoporos Int 23:2769–2774
Lorentzon M, Branco J, Brandi ML et al (2019) Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther 36:2811–2824
Johansson H, Odén A, Kanis JA, McCloskey EV, Morris HA, Cooper C, Vasikaran S (2014) A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif Tissue Int 94:560–567
Kanis JA, Johansson H, Harvey NC et al (2021) The use of 2-, 5-, and 10-year probabilities to characterize fracture risk after a recent sentinel fracture. Osteoporos Int 32:47–54
McCloskey EV, Borgström F, Cooper C, Harvey NC, Javaid MK, Lorentzon M, JA. K (2021) Short time horizons for fracture prediction tools: time for a rethink. Osteoporos Int in press
Kanis JA, Harvey NC, Cooper C, Johansson H, Odén A, McCloskey EV (2016) A systematic review of intervention thresholds based on FRAX : a report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos 11:25
Hippisley-Cox J, Coupland C (2009) Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. 339:b4229
National Institute for Health and Care Excellence (2012) Osteoporosis: assessing the risk of fragility fracture. NICE clinical guideline [CG146]. Manchester: NICE. www.nice.org.uk/guidance/cg146
National Institute for Health and Care Excellence (NICE) (2017) Osteoporosis: Quality standard [QS149]. Manchester, NICE
Kanis JA, Johansson H, Oden A, McCloskey EV (2011) Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int 22:809–816
Leslie WD, Morin S, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA (2012) Fracture risk assessment without bone density measurement in routine clinical practice. Osteoporos Int 23:75–85
Johansson H, Kanis JA, Odén A et al (2014) Impact of femoral neck and lumbar spine BMD discordances on FRAX probabilities in women: a meta-analysis of international cohorts. Calcified Tiss Inter 95:428–435
McCloskey EV, Odén A, Harvey NC et al (2016) A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Mineral Res 31:940–948
Leslie WD, Lix LM, Morin SN, Johansson H, Odén A, McCloskey EV, Kanis JA (2016) Adjusting Hip Fracture Probability in Men and Women Using Hip Axis Length: the Manitoba Bone Density Database. J Clin Densitom 19:326–331
Masud T, Binkley N, Boonen S, Hannan MT (2011) Official Positions for FRAX® clinical regarding falls and frailty: can falls and frailty be used in FRAX®? From Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX®. J Clin Densitom 14:194–204
Johansson H, Odén A, Lorentzon M, McCloskey E, Kanis JA, Harvey NC, Karlsson MK, Mellström D (2015) Is the Swedish FRAX model appropriate for Swedish immigrants? Osteoporos Int 26:2617–2622
Leslie WD, Johansson H, McCloskey EV, Harvey NC, Kanis JA, Hans D (2018) Comparison of Methods for Improving Fracture Risk Assessment in Diabetes: The Manitoba BMD Registry. J Bone Mineral Res 33:1923–1930
Brennan SL, Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA (2014) FRAX provides robust fracture prediction regardless of socioeconomic status. Osteoporos Int 25:61–69
Leslie WD, Orwoll ES, Nielson CM, Morin SN, Majumdar SR, Johansson H, Odén A, McCloskey EV, Kanis JA (2014) Estimated lean mass and fat mass differentially affect femoral bone density and strength index but are not FRAX independent risk factors for fracture. J Bone Mineral Res 29:2511–2519
Whitlock RH, Leslie WD, Shaw J, Rigatto C, Thorlacius L, Komenda P, Collister D, Kanis JA, Tangri N (2019) The Fracture Risk Assessment Tool (FRAX®) predicts fracture risk in patients with chronic kidney disease. Kidney Int 95:447–454
Baruch Fischhoff, Noel T. Brewer, Downs. JS (2018) Communicating risks and benefits: an evidence-based user's guide. US Department of Health and Human Services Food and Drug Administration.,
Fink HA, Milavetz DL, Palermo L, Nevitt MC, Cauley JA, Genant HK, Black DM, Ensrud KE (2005) What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res 20:1216–1222
Melton LJ 3rd, Atkinson EJ, Cooper C, O’Fallon WM, Riggs BL (1999) Vertebral fractures predict subsequent fractures. Osteoporos Int 10:214–221
Lindsay R, Silverman SL, Cooper C et al (2001) Risk of new vertebral fracture in the year following a fracture. JAMA 285:320–323
Jang HD, Kim EH, Lee JC, Choi SW, Kim K, Shin BJ (2020) Current concepts in the management of osteoporotic vertebral fractures: a narrative review. Asian Spine J 14:898–909
Lewiecki EM (2010) Bone densitometry and vertebral fracture assessment. Curr Osteoporos Rep 8:123–130
Gausden EB, Nwachukwu BU, Schreiber JJ, Lorich DG, Lane JM (2017) Opportunistic use of CT imaging for osteoporosis screening and bone density assessment: a qualitative systematic review. J Bone Joint Surg Am 99:1580–1590
Bromiley PA, Clark EM, Poole KE (2020) Computer-aided diagnostic systems for osteoporotic vertebral fracture detection: opportunities and challenges. J Bone Mineral Research 35:2305–2306
Kolanu N, Silverstone EJ, Ho BH, Pham H, Hansen A, Pauley E, Quirk AR, Sweeney SC, Center JR, Pocock NA (2020) Clinical utility of computer-aided diagnosis of vertebral fractures from computed tomography images. J Bone Miner Res 35:2307–2312
Howlett DC, Drinkwater KJ, Mahmood N, Illes J, Griffin J, Javaid K (2020) Radiology reporting of osteoporotic vertebral fragility fractures on computed tomography studies: results of a UK national audit. Eur Radiol 30:4713–4723
Kim YM, Demissie S, Genant HK, Cheng X, Yu W, Samelson EJ, Kiel DP, Bouxsein ML (2012) Identification of prevalent vertebral fractures using CT lateral scout views: a comparison of semi-automated quantitative vertebral morphometry and radiologist semi-quantitative grading. Osteoporos Int 23:1007–1016
Rubin KH, Rothmann MJ, Holmberg T et al (2018) Effectiveness of a two-step population-based osteoporosis screening program using FRAX: the randomized Risk-stratified Osteoporosis Strategy Evaluation (ROSE) study. Osteoporos Int 29:567–578
Merlijn T, Swart KM, van Schoor NM et al (2019) The effect of a screening and treatment program for the prevention of fractures in older women: a randomized pragmatic trial. J Bone Miner Res 34:1993–2000
Merlijn T, Swart KMA, van der Horst HE, Netelenbos JC, Elders PJM (2020) Fracture prevention by screening for high fracture risk: a systematic review and meta-analysis. Osteoporos Int 31:251–257
Ismail AA, Cooper C, Felsenberg D, Varlow J, Kanis JA, Silman AJ, O’Neill TW (1999) Number and type of vertebral deformities: epidemiological characteristics and relation to back pain and height loss. European Vertebral Osteoporosis Study Group. Osteoporos Int 9:206–213
Zhang Q, Dong J, Zhou D, Liu F (2020) Comparative risk of fracture for bariatric procedures in patients with obesity: a systematic review and Bayesian network meta-analysis. Int J Surg (London, England) 75:13–23
Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A (2008) Case finding for the management of osteoporosis with FRAX–assessment and intervention thresholds for the UK. Osteoporos Int 19:1395–1408
National Institute for Health and Care Excellence (2019) Bisphosphonates for treating osteoporosis. Technology appraisal guidance [TA464]. https://www.nice.org.uk/guidance/ta464
McCloskey E, Kanis JA, Johansson H et al (2015) FRAX-based assessment and intervention thresholds–an exploration of thresholds in women aged 50 years and older in the UK. Osteoporos Int 26:2091–2099
Shepstone L, Lenaghan E, Cooper C et al (2018) Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet 391:741–747
Cosman F, Crittenden DB, Adachi JD et al (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377:1417–1427
Kendler DL, Marin F, Zerbini CAF et al (2018) Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 391:230–240
Kanis JA, Harvey NC, McCloskey E et al (2020) Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int 31:1–12
Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, Adachi JD (2003) Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone 33:522–532
Kanis JA, Cooper C, Rizzoli R, Reginster JY (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30:3–44
Johnell O, Oden A, Caulin F, Kanis JA (2001) Acute and long-term increase in fracture risk after hospitalization for vertebral fracture. Osteoporos Int 12:207–214
Giangregorio LM, Leslie WD (2010) Time since prior fracture is a risk modifier for 10-year osteoporotic fractures. J Bone Miner Res 25:1400–1405
Dretakis KE, Dretakis EK, Papakitsou EF, Psarakis S, Steriopoulos K (1998) Possible predisposing factors for the second hip fracture. Calcif Tissue Int 62:366–369
Nymark T, Lauritsen JM, Ovesen O, Röck ND, Jeune B (2006) Short time-frame from first to second hip fracture in the Funen County Hip Fracture Study. Osteoporos Int 17:1353–1357
Ryg J, Rejnmark L, Overgaard S, Brixen K, Vestergaard P (2009) Hip fracture patients at risk of second hip fracture: a nationwide population-based cohort study of 169,145 cases during 1977–2001. J Bone Miner Res 24:1299–1307
van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ (2009) Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis 68:99–102
Johansson H, Siggeirsdóttir K, Harvey NC, Odén A, Gudnason V, McCloskey E, Sigurdsson G, Kanis JA (2017) Imminent risk of fracture after fracture. Osteoporos Int 28:775–780
Kanis JA, Johansson H, Harvey NC, Gudnason V, Sigurdsson G, Siggeirsdottir K, Lorentzon M, Liu E, Vandenput L, McCloskey EV (2021) The effect on subsequent fracture risk of age, sex, and prior fracture site by recency of prior fracture. Osteoporosis International
Body JJ, Marin F, Kendler DL et al (2020) Efficacy of teriparatide compared with risedronate on FRAX(®)-defined major osteoporotic fractures: results of the VERO clinical trial. Osteoporos Int 31:1935–1942
Lyles KW, Colon-Emeric CS, Magaziner JS et al (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809
Kanis JA, Johansson H, Harvey NC, Lorentzon M, Liu E, Vandenput L, EV. M, (2021) An assessment of intervention thresholds for very high fracture risk applied to the NOGG guidelines. Osteoporos Int 32:1951–1960
Kanis JA, Rizzoli R, Cooper C, Reginster JY (2014) Challenges for the development of bone-forming agents in Europe. Calcif Tissue Int 94:469–473
Johansson H, Kanis JA, Oden A, Compston J, McCloskey E (2012) A comparison of case-finding strategies in the UK for the management of hip fractures. Osteoporos Int 23:907–915
Cummings SR, Black DM, Thompson DE et al (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280:2077–2082
Eastell R, Black DM, Boonen S et al (2009) Effect of once-yearly zoledronic acid five milligrams on fracture risk and change in femoral neck bone mineral density. J Clin Endocrinol Metab 94:3215–3225
Harvey NC, Kanis JA, Odén A, Nakamura T, Shiraki M, Sugimoto T, Kuroda T, Johansson H, McCloskey EV (2015) Efficacy of weekly teriparatide does not vary by baseline fracture probability calculated using FRAX. Osteoporos Int 26:2347–2353
McCloskey E (2016) A BMD threshold for treatment efficacy in osteoporosis? A need to consider the whole evidence base. Osteoporos Int 27:417–419
Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Wong S, Wiessing KR, Bolland MJ, Bastin S, Gamble GD (2018) Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med 379:2407–2416
Johansson H, Oden A, Johnell O, Jonsson B, de Laet C, Oglesby A, McCloskey EV, Kayan K, Jalava T, Kanis JA (2004) Optimization of BMD measurements to identify high risk groups for treatment–a test analysis. J Bone Mineral Res 19:906–913
Leslie WD, Majumdar SR, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA (2012) High fracture probability with FRAX usually indicates densitometric osteoporosis: implications for clinical practice. Osteoporos Int 23:391–397
Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA (2012) Does osteoporosis therapy invalidate FRAX for fracture prediction? J Bone Mineral Research 27:1243–1251
Fabiani R, Naldini G, Chiavarini M (2019) Dietary patterns in relation to low bone mineral density and fracture risk: a systematic review and meta-analysis. Advances in nutrition (Bethesda, Md) 10:219–236
Lin P-H, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, Barclay D, Svetkey LP (2003) The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr 133:3130–3136
Groenendijk I, den Boeft L, van Loon LJC, de Groot L (2019) High versus low dietary protein intake and bone health in older adults: a systematic review and meta-analysis. Comput Struct Biotechnol J 17:1101–1112
Darling AL, Manders RJF, Sahni S, Zhu K, Hewitt CE, Prince RL, Millward DJ, Lanham-New SA (2019) Dietary protein and bone health across the life-course: an updated systematic review and meta-analysis over 40 years. Osteoporos Int 30:741–761
Myint MW, Wu J, Wong E, Chan SP, To TS, Chau MW, Ting KH, Fung PM, Au KS (2013) Clinical benefits of oral nutritional supplementation for elderly hip fracture patients: a single blind randomised controlled trial. Age Ageing 42:39–45
Iguacel I, Miguel-Berges ML, Gómez-Bruton A, Moreno LA, Julián C (2019) Veganism, vegetarianism, bone mineral density, and fracture risk: a systematic review and meta-analysis. Nutr Rev 77:1–18
Tong TYN, Appleby PN, Armstrong MEG, Fensom GK, Knuppel A, Papier K, Perez-Cornago A, Travis RC, Key TJ (2020) Vegetarian and vegan diets and risks of total and site-specific fractures: results from the prospective EPIC-Oxford study. BMC Med 18:353
Department of Health (1991) Dietary reference values for food energy and nutrients for the United Kingdom. HMSO, London
Scientific Advisory Committee on Nutrition (SACN) (2016) Vitamin D and Health. https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition
Royal Osteoporosis Society (2018) Vitamin D and bone health: a practical clinical guideline for patient management. https://strwebprdmedia.blob.core.windows.net/media/ef2ideu2/ros-vitamin-d-and-bone-health-in-adults-february-2020.pdf
Yao P, Bennett D, Mafham M, Lin X, Chen Z, Armitage J, Clarke R (2019) Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw Open 2:e1917789
Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A (2007) Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 370:657–666
Group. DVDIPAoRT (2010) Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. Bmj 340:b5463
Harvey NC, Biver E, Kaufman JM et al (2017) The role of calcium supplementation in healthy musculoskeletal ageing. Osteoporos Int 28:447–462
Kahwati LC, Weber RP, Pan H, Gourlay M, LeBlanc E, Coker-Schwimmer M, Viswanathan M (2018) Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 319:1600–1612
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC (2010) Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 303:1815–1822
Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A (2016) Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med 176:175–183
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G (2011) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev Cd000333
Kemmler W, Shojaa M, Kohl M, von Stengel S (2020) Effects of different types of exercise on bone mineral density in postmenopausal women: a systematic review and meta-analysis. Calcif Tissue Int 107:409–439
Babatunde OO, Bourton AL, Hind K, Paskins Z, Forsyth JJ (2020) Exercise interventions for preventing and treating low bone mass in the forearm: a systematic review and meta-analysis. Arch Phys Med Rehabil 101:487–511
Kelley GA, Kelley KS, Kohrt WM (2013) Exercise and bone mineral density in men: a meta-analysis of randomized controlled trials. Bone 53:103–111
Royal Osteoporosis Society (2018) Strong, Steady and Straight; An Expert Consensus Statement on physical activity and exercise for osteoporosis. https://theros.org.uk/media/0o5h1l53/ros-strong-steady-straight-quick-guide-february-2019.pdf
Kunutsor SK, Leyland S, Skelton DA, James L, Cox M, Gibbons N, Whitney J, Clark EM (2018) Adverse events and safety issues associated with physical activity and exercise for adults with osteoporosis and osteopenia: a systematic review of observational studies and an updated review of interventional studies. Journal of frailty, sarcopenia and falls 3:155–178
Bhasin S, Gill TM, Reuben DB et al (2020) A Randomized Trial of a Multifactorial Strategy to Prevent Serious Fall Injuries. N Engl J Med 383:129–140
Lamb SE, Bruce J, Hossain A et al (2020) Screening and Intervention to Prevent Falls and Fractures in Older People. N Engl J Med 383:1848–1859
El-Khoury F, Cassou B, Charles M-A, Dargent-Molina P (2013) The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. 347:f6234
Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K, Clemson L, Hopewell S, Lamb SE (2019) Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev 1:Cd012424
Zhao R, Bu W, Chen X (2019) The efficacy and safety of exercise for prevention of fall-related injuries in older people with different health conditions, and differing intervention protocols: a meta-analysis of randomized controlled trials. BMC Geriatr 19:341
Sherrington C, Michaleff ZA, Fairhall N, Paul SS, Tiedemann A, Whitney J, Cumming RG, Herbert RD, Close JCT, Lord SR (2017) Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med 51:1750–1758
Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, Lamb SE (2012) Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev Cd007146
Jepsen DB, Thomsen K, Hansen S, Jørgensen NR, Masud T, Ryg J (2017) Effect of whole-body vibration exercise in preventing falls and fractures: a systematic review and meta-analysis. BMJ Open 7:e018342
Thorin MH, Wihlborg A, Åkesson K, Gerdhem P (2016) Smoking, smoking cessation, and fracture risk in elderly women followed for 10 years. Osteoporos Int 27:249–255
Shen GS, Li Y, Zhao G, Zhou HB, Xie ZG, Xu W, Chen HN, Dong QR, Xu YJ (2015) Cigarette smoking and risk of hip fracture in women: a meta-analysis of prospective cohort studies. Injury 46:1333–1340
Min W, An R, Li S, Feng J, Yang J, Huang Z (2017) The effects of preoperative smoking cessation on the healing of fractures and postoperative complications: a systematic review and meta-analysis. Biomed Res 28:1883–1889
Song TH, Shim JC, Jung DU, Moon JJ, Jeon DW, Kim SJ, Oh MK (2018) Increased bone mineral density after abstinence in male patients with alcohol dependence. Clin Psychopharmacol Neurosci 16:282–289
Health. Do (2016) UK Chief Medical Officers’ Alcohol Guidelines Review Summary of the proposed new guidelines. Williams Lea
Crandall CJ, Newberry SJ, Diamant A, Lim YW, Gellad WF, Booth MJ, Motala A, Shekelle PG (2014) Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med 161:711–723
Black DM, Cummings SR, Karpf DB et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541
Orwoll E, Ettinger M, Weiss S et al (2000) Alendronate for the treatment of osteoporosis in men. N Engl J Med 343:604–610
Saag KG, Emkey R, Schnitzer TJ et al (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 339:292–299
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
Reginster J, Minne HW, Sorensen OH et al (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11:83–91
Boonen S, Orwoll ES, Wenderoth D, Stoner KJ, Eusebio R, Delmas PD (2009) Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res 24:719–725
Wallach S, Cohen S, Reid DM et al (2000) Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int 67:277–285
Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, Eusebio RA, Devogelaer JP (2000) Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res 15:1006–1013
Delmas PD, Recker RR, Chesnut CH 3rd, Skag A, Stakkestad JA, Emkey R, Gilbride J, Schimmer RC, Christiansen C (2004) Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 15:792–798
Chesnut CH 3rd, Skag A, Christiansen C et al (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19:1241–1249
Reginster JY, Adami S, Lakatos P et al (2006) Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis 65:654–661
Eisman JA, Civitelli R, Adami S et al (2008) Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol 35:488–497
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Boonen S, Reginster JY, Kaufman JM et al (2012) Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 367:1714–1723
Reid DM, Devogelaer JP, Saag K et al (2009) Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 373:1253–1263
Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Bastin S, Gamble GD (2020) Effects of zoledronate on cancer, cardiac events, and mortality in osteopenic older women. J Bone Miner Res 35:20–27
Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM (2010) Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab 95:4380–4387
Saag KG, Wagman RB, Geusens P, Adachi JD, Messina OD, Emkey R, Chapurlat R, Wang A, Pannacciulli N, Lems WF (2018) Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 6:445–454
Cummings SR, San Martin J, McClung MR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Bone HG, Wagman RB, Brandi ML et al (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5:513–523
Langdahl BL, Teglbjaerg CS, Ho PR et al (2015) A 24-month study evaluating the efficacy and safety of denosumab for the treatment of men with low bone mineral density: results from the ADAMO trial. J Clin Endocrinol Metab 100:1335–1342
(2014) https://www.gov.uk/drug-safety-update/denosumab-monitoring-recommended Accessed January 7th 2021
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96:972–980
Miller PD, Wagman RB, Peacock M, Lewiecki EM, Bolognese MA, Weinstein RL, Ding B, San Martin J, McClung MR (2011) Effect of denosumab on bone mineral density and biochemical markers of bone turnover: six-year results of a phase 2 clinical trial. J Clin Endocrinol Metab 96:394–402
Tsourdi E, Zillikens MC, Meier C, et al. (2020) Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. The Journal of clinical endocrinology and metabolism
Cummings SR, Ferrari S, Eastell R et al (2018) Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res 33:190–198
Dennison EM, Cooper C, Kanis JA, Bruyère O, Silverman S, McCloskey E, Abrahamsen B, Prieto-Alhambra D, Ferrari S (2019) Fracture risk following intermission of osteoporosis therapy. Osteoporos Int 30:1733–1743
Reid IR, Horne AM, Mihov B, Gamble GD (2017) Bone loss after denosumab: only partial protection with zoledronate. Calcif Tissue Int 101:371–374
Horne AM, Mihov B, Reid IR (2019) Effect of zoledronate on bone loss after romosozumab/denosumab: 2-year follow-up. Calcif Tissue Int 105:107–108
Anastasilakis AD, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Makras P (2019) Zoledronate for the prevention of bone loss in women discontinuing denosumab treatment. A Prospective 2-Year Clinical Trial. J Bone Mineral Res; 34:2220–2228
Everts-Graber J, Reichenbach S, Ziswiler HR, Studer U, Lehmann T (2020) A single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of bone mass gains. J Bone Miner Res 35:1207–1215
Sølling AS, Harsløf T, Langdahl B (2020) Treatment with zoledronate subsequent to denosumab in osteoporosis: a randomized trial. J Bone Miner Res 35:1858–1870
Makras P, Appelman-Dijkstra NM, Papapoulos SE, van Wissen S, Winter EM, Polyzos SA, Yavropoulou MP, Anastasilakis AD (2021) The duration of denosumab treatment and the efficacy of zoledronate to preserve bone mineral density after its discontinuation. J Clin Endocrinol Metab 106:e4155–e4162
Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Zillikens MC (2017) Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone 105:11–17
Kendler D, Chines A, Clark P, Ebeling PR, McClung M, Rhee Y, Huang S, Stad RK (2020) Bone mineral density after transitioning from denosumab to alendronate. J Clin Endocrinol Metab 105:e255-264
Marjoribanks J, Farquhar C, Roberts H, Lethaby A (2012) Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev CD004143
Rossouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Gartlehner G, Patel SV, Feltner C, Weber RP, Long R, Mullican K, Boland E, Lux L, Viswanathan M (2017) Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: evidence report and systematic review for the US Preventive Services Task Force. JAMA 318:2234–2249
Rozenberg S, Al-Daghri N, Aubertin-Leheudre M et al (2020) Is there a role for menopausal hormone therapy in the management of postmenopausal osteoporosis? Osteoporos Int 31:2271–2286
Gallagher JC, Goldgar D (1990) Treatment of postmenopausal osteoporosis with high doses of synthetic calcitriol. A randomized controlled study. Ann Intern Med 113:649–655
Ettinger B, Black DM, Mitlak BH et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282:637–645
Barrett-Connor E, Cox DA, Song J, Mitlak B, Mosca L, Grady D (2009) Raloxifene and risk for stroke based on the framingham stroke risk score. Am J Med 122:754–761
Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK, Use R, for The Heart Trial I, (2006) Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 355:125–137
Meunier PJ, Roux C, Seeman E et al (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350:459–468
Reginster JY, Seeman E, De Vernejoul MC et al (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90:2816–2822
Kaufman JM, Audran M, Bianchi G et al (2013) Efficacy and safety of strontium ranelate in the treatment of osteoporosis in men. J Clin Endocrinol Metab 98:592–601
Osborne V, Layton D, Perrio M, Wilton L, Shakir SA (2010) Incidence of venous thromboembolism in users of strontium ranelate: an analysis of data from a prescription-event monitoring study in England. Drug Saf 33:579–591
Agency EM (2013) PSUR assessment report Strontium Ranelate
Blake GM, Compston JE, Fogelman I (2009) Could strontium ranelate have a synergistic role in the treatment of osteoporosis? J Bone Miner Res 24:1354–1357
Neer RM, Arnaud CD, Zanchetta JR et al (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Diez-Perez A, Marin F, Eriksen EF, Kendler DL, Krege JH, Delgado-Rodriguez M (2019) Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: a systematic review and meta-analysis. Bone 120:1–8
Simpson EL, Martyn-St James M, Hamilton J, Wong R, Gittoes N, Selby P, Davis S (2020) Clinical effectiveness of denosumab, raloxifene, romosozumab, and teriparatide for the prevention of osteoporotic fragility fractures: a systematic review and network meta-analysis. Bone 130:115081
Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA (2003) The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17
Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, Krege JH, Krohn K, Warner MR (2009) Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum 60:3346–3355
Kendler DL, Bone HG, Massari F et al (2019) Bone mineral density gains with a second 12-month course of romosozumab therapy following placebo or denosumab. Osteoporos Int 30:2437–2448
Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, Rosen CJ (2000) Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab 85:2129–2134
Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, Burnett-Bowie SA (2015) Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet 386:1147–1155
Cosman F, Crittenden DB, Ferrari S, Khan A, Lane NE, Lippuner K, Matsumoto T, Milmont CE, Libanati C, Grauer A (2018) FRAME study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Mineral Res 33:1219–1226
McClung MR, Brown JP, Diez-Perez A et al (2018) Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J Bone Miner Res 33:1397–1406
Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, Ebeling PR, Adachi JD, Miyauchi A, Gielen E, Milmont CE, Libanati C, Grauer A (2019) One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME extension study. J Bone Miner Research 34:419–428
Langdahl BL, Libanati C, Crittenden DB et al (2017) Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 390:1585–1594
Keaveny TM, Crittenden DB, Bolognese MA et al (2017) Greater gains in spine and hip strength for romosozumab compared with teriparatide in postmenopausal women with low bone mass. J Bone Miner Research 32:1956–1962
Ebina K, Hirao M, Tsuboi H, et al. (2020) Effects of prior osteoporosis treatment on early treatment response of romosozumab in patients with postmenopausal osteoporosis. Bone 140:115574
Nayak S, Greenspan SL (2019) A systematic review and meta-analysis of the effect of bisphosphonate drug holidays on bone mineral density and osteoporotic fracture risk. Osteoporos Int 30:705–720
National Institute for Health and Care Excellence (2016) Multimorbidity: clinical assessment and management. NICE guideline [NG56]. https://www.nice.org.uk/guidance/ng56
Adler RA, El-Hajj Fuleihan G, Bauer DC et al (2016) Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Research 31:16–35
Fink HA, MacDonald R, Forte ML et al (2019) Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med 171:37–50
Ensrud KE, Barrett-Connor EL, Schwartz A et al (2004) Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Min Res 19:1259–1269
Black DM, Schwartz AV, Ensrud KE et al (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
Ravn P, Christensen JO, Baumann M, Clemmesen B (1998) Changes in biochemical markers and bone mass after withdrawal of ibandronate treatment: prediction of bone mass changes during treatment. Bone 22:559–564
Watts NB, Chines A, Olszynski WP, McKeever CD, McClung MR, Zhou X, Grauer A (2008) Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int 19:365–372
Black DM, Reid IR, Boonen S et al (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27:243–254
Kim TY, Bauer DC, McNabb BL, Schafer AL, Cosman F, Black DM, Eastell R (2019) Comparison of BMD changes and bone formation marker levels 3 years after bisphosphonate discontinuation: FLEX and HORIZON-PFT Extension I Trials. J Bone Miner Res 34:810–816
Bauer DC, Schwartz A, Palermo L, Cauley J, Hochberg M, Santora A, Cummings SR, Black DM (2014) Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern Med 174:1126–1134
Khan AA, Morrison A, Hanley DA et al (2015) Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res 30:3–23
Khosla S, Burr D, Cauley J et al (2007) Bisphosphonate-associated osteonecrosis of the jaw: report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res 22:1479–1491
Scottish Dental Clinical Effectiveness Programme (2017) Oral health management of patients at risk of medication-related osteonecrosis of the jaw: dental clinical guidance. https://www.sdcep.org.uk/wp-content/uploads/2017/04/SDCEP-Oral-Health-Management-of-Patients-at-Risk-of-MRONJ-Guidance-full.pdf
Salzman R, Hoza J, Perina V, Stárek I (2013) Osteonecrosis of the external auditory canal associated with oral bisphosphonate therapy: case report and literature review. Otol Neurotol 34:209–213
Black DM, Abrahamsen B, Bouxsein ML, Einhorn T, Napoli N (2019) Atypical femur fractures: review of epidemiology, relationship to bisphosphonates, prevention, and clinical management. Endocr Rev 40:333–368
Shane E, Burr D, Ebeling PR et al (2010) Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Min Research 25:2267–2294
Shane E, Burr D, Abrahamsen B et al (2014) Atypical subtrochanteric and diaphyseal femoral fractures: second report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res 29:1–23
Gedmintas L, Solomon DH, Kim SC (2013) Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. J Bone Min Research 28:1729–1737
Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R (2016) Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. Bmj 353:i3365
van de Laarschot DM, McKenna MJ, Abrahamsen B, Langdahl B, Cohen-Solal M, Guañabens N, Eastell R, Ralston SH, Zillikens MC (2020) Medical management of patients after atypical femur fractures: a systematic review and recommendations from the European Calcified Tissue Society. J Clin Endocrin Metab 105:1682–1699
Carter M (2019) Prevention of glucocorticoid-induced osteoporosis: clinical audit to evaluate the implementation of National Osteoporosis Guideline Group 2017 guidelines in a primary care setting. J Clin Densitom 22:25–30
van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Adachi JD, Saag KG, Delmas PD et al (2001) Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 44:202–211
Cohen S, Levy RM, Keller M et al (1999) Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 42:2309–2318
Deng J, Silver Z, Huang E, Zheng E, Kavanagh K, Wen A, Cheng W, Dobransky J, Sanger S, Grammatopoulos G (2020) Pharmacological prevention of fractures in patients undergoing glucocorticoid therapies: a systematic review and network meta-analysis. Rheumatology (Oxford)
Brown JE, Handforth C, Compston JE, et al. (2020) Guidance for the assessment and management of prostate cancer treatment-induced bone loss. A consensus position statement from an expert group. J Bone Oncol 25:100311
Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, Finkelstein JS, Endocrine S (2012) Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97:1802–1822
Health NIf (2018) Osteoporosis in Men
Mittan D, Lee S, Miller E, Perez RC, Basler JW, Bruder JM (2002) Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. J Clin Endocrinol Metab 87:3656–3661
Shahinian VB, Kuo YF, Freeman JL, Goodwin JS (2005) Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 352:154–164
Joseph JS, Lam V, Patel MI (2019) Preventing osteoporosis in men taking androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Eur Urol Oncol 2:551–561
Poon Y, Pechlivanoglou P, Alibhai SMH, Naimark D, Hoch JS, Papadimitropoulos E, Hogan ME, Krahn M (2018) Systematic review and network meta-analysis on the relative efficacy of osteoporotic medications: men with prostate cancer on continuous androgen-deprivation therapy to reduce risk of fragility fractures. BJU Int 121:17–28
Reid DM, Doughty J, Eastell R, Heys SD, Howell A, McCloskey EV, Powles T, Selby P, Coleman RE (2008) Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev 34(Suppl 1):S3-18
Hadji P, Aapro MS, Body JJ et al (2017) Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol 7:1–12
Excellence NIfHaC (2017) Early breast cancer (preventing recurrence and improving survival): adjuvant bisphosphonates
Waqas K, Lima Ferreira J, Tsourdi E, Body JJ, Hadji P, Zillikens MC (2021) Updated guidance on the management of cancer treatment-induced bone loss (CTIBL) in pre- and postmenopausal women with early-stage breast cancer. J Bone Oncol 28:100355
Miyashita H, Satoi S, Kuno T, Cruz C, Malamud S, Kim SM (2020) Bone modifying agents for bone loss in patients with aromatase inhibitor as adjuvant treatment for breast cancer; insights from a network meta-analysis. Breast Cancer Res Treat 181:279–289
World Health Organization (1987) Traitement de la douleur cancéreuse. Genève: Organisation mondiale de la Santé. https://apps.who.int/iris/handle/10665/41712
Schofield P (2018) The Assessment of pain in older people: UK National Guidelines. Age Ageing 47:i1–i22
British Geriatric Society (2013) Guidance on the management of pain in older people. Age Ageing 42:i1–i57
Knopp-Sihota JA, Newburn-Cook CV, Homik J, Cummings GG, Voaklander D (2012) Calcitonin for treating acute and chronic pain of recent and remote osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Osteoporos Int 23:17–38
European Medicines Agency (2012) European Medicines Agency recommends limiting long-term use of calcitonin medicines. https://www.ema.europa.eu/en/news/european-medicines-agency-recommends-limiting-long-term-use-calcitonin-medicines
Armingeat T, Brondino R, Pham T, Legré V, Lafforgue P (2006) Intravenous pamidronate for pain relief in recent osteoporotic vertebral compression fracture: a randomized double-blind controlled study. Osteoporos Int 17:1659–1665
Gibbs JC, MacIntyre NJ, Ponzano M, Templeton JA, Thabane L, Papaioannou A, Giangregorio LM (2019) Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database of Systematic Reviews
Ebeling PR, Akesson K, Bauer DC et al (2019) The efficacy and safety of vertebral augmentation: a second ASBMR Task Force Report 34:3–21
Svensson HK, Olsson LE, Hansson T, Karlsson J, Hansson-Olofsson E (2017) The effects of person-centered or other supportive interventions in older women with osteoporotic vertebral compression fractures-a systematic review of the literature. Osteoporos Int 28:2521–2540
Hofler RC, Jones GA (2020) Bracing for acute and subacute osteoporotic compression fractures: a systematic review of the literature. World Neurosurg 141:e453–e460
Buchbinder R, Johnston RV, Rischin KJ, Homik J, Jones CA, Golmohammadi K, Kallmes DF (2018) Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database Syst Rev 11:Cd006349
Zambito A, Bianchini D, Gatti D, Rossini M, Adami S, Viapiana O (2007) Interferential and horizontal therapies in chronic low back pain due to multiple vertebral fractures: a randomized, double blind, clinical study. Osteoporos Int 18:1541–1545
Axelsson KF, Johansson H, Lundh D, Möller M, Lorentzon M (2020) Association between recurrent fracture risk and implementation of fracture liaison services in four Swedish hospitals: a cohort study. J Bone Miner Res 35:1216–1223
Wu CH, Tu ST, Chang YF, Chan DC, Chien JT, Lin CH, Singh S, Dasari M, Chen JF, Tsai KS (2018) Fracture liaison services improve outcomes of patients with osteoporosis-related fractures: a systematic literature review and meta-analysis. Bone 111:92–100
Department of Health (2009) Fracture prevention services - an economic evaluation
NHS_RightCare (2017) Falls and Fragility Fractures Pathway. https://www.england.nhs.uk/rightcare/products/pathways/falls-and-fragility-fractures-pathway/
Javaid MK, Kyer C, Mitchell PJ et al (2015) Effective secondary fracture prevention: implementation of a global benchmarking of clinical quality using the IOF Capture the Fracture(R) Best Practice Framework tool. Osteoporos Int 26:2573–2578
Royal Osteoporosis Society (2019) Effective Secondary Prevention of Fragility Fractures: Clinical Standards for Fracture Liaison Services https://theros.org.uk/media/1eubz33w/ros-clinical-standards-for-fracture-liaison-services-august-2019.pdf
Ganda K, Puech M, Chen JS, Speerin R, Bleasel J, Center JR, Eisman JA, March L, Seibel MJ (2013) Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 24:393–406
Ganda K, Mitchell PJ, Seibel MJ (2019) Chapter 3, Models of secondary fracture prevention: systematic review and metaanalysis of outcomes. In Mitchell PJ, Seibel MJ (eds) Secondary Fracture Prevention, an International Perspective. Elsevier Inc, pp 33–62
Wu CH, Chen CH, Chen PH, Yang JJ, Chang PC, Huang TC, Bagga S, Sharma Y, Lin RM, Chan DC (2018) Identifying characteristics of an effective fracture liaison service: systematic literature review. Osteoporos Int 29:1023–1047
Public Health England (2017) Falls and fracture consensus statement: Supporting commissioning for prevention Public Health England with the National Falls Prevention Coordination Group,www.gov.uk/phe
Royal Osteoporosis Society (2017) Clinical Guidance for the Effective Identification of Vertebral Fractures https://theros.org.uk/healthcare-professionals/tools-and-resources/clinical-guidance/
Laurna Bullock, Fay Crawford-Manning, Elizabeth Cottrell, et al. (2021) Co-designing a model Fracture Liaison Service consultation with patients, carers and clinicians: a Delphi survey to inform the content of the i-FraP complex consultation intervention. Archives in Osteoporosis in press:
Paskins Z, Torres Roldan VD, Hawarden AW, et al. (2020) Quality and effectiveness of osteoporosis treatment decision aids: a systematic review and environmental scan. Osteoporos Int
National Institute for Health and Care Excellence (2009) Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. Clinical Guideline [CG76]. https://www.nice.org.uk/guidance/cg76
Cornelissen D, de Kunder S, Si L, Reginster JY, Evers S, Boonen A, Hiligsmann M (2020) Interventions to improve adherence to anti-osteoporosis medications: an updated systematic review. Osteoporos Int 31:1645–1669
Martin J, Viprey M, Castagne B, Merle B, Giroudon C, Chapurlat R, Schott AM (2020) Interventions to improve osteoporosis care: a systematic review and meta-analysis. Osteoporos Int 31:429–446
Clynes MA, Westbury LD, Dennison EM, Kanis JA, Javaid MK, Harvey NC, Fujita M, Cooper C, Leslie WD, Shuhart CR (2020) Bone densitometry worldwide: a global survey by the ISCD and IOF. Osteoporos Int 31:1779–1786
Foundation TH (2021) Quality improvement made simple: what everyone should know about health care quality improvement. 3rd ednThe Health Foundation, 8 Salisbury Square, London
Javaid MK, Harvey NC, McCloskey E, Kanis JA, C C (2021) Assessment and management of imminent fracture risk in the setting of the fracture liaison service. Osteo Internat in press:
Andrews J, Guyatt G, Oxman AD et al (2013) GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 66:719–725
Jaeschke R, Guyatt GH, Dellinger P, Schünemann H, Levy MM, Kunz R, Norris S, Bion J (2008) Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. 337:a744
National Institute for Health and Care Excellence (2012) The guidelines manual. Process and methods [PMG6]. Section 9 Developing and wording guideline recommendations
Nayak S, Greenspan SL (2017) Osteoporosis treatment efficacy for men: a systematic review and meta-analysis. J Am Geriatr Soc 65:490–495
Zeng LF, Pan BQ, Liang GH et al (2019) Does routine anti-osteoporosis medication lower the risk of fractures in male subjects? An updated systematic review with meta-analysis of clinical trials. Front Pharmacol 10:882
Wang YK, Zhang YM, Qin SQ, Wang X, Ma T, Guo JB, Zhu C, Luo ZJ (2018) Effects of alendronate for treatment of glucocorticoid-induced osteoporosis: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 97:e12691
Yanbeiy ZA, Hansen KE (2019) Denosumab in the treatment of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis. Drug Des Devel Ther 13:2843–2852
Wändell P, Li X, Carlsson AC, Sundquist J, Sundquist K (2021) Osteoporotic fractures among foreign-born individuals: a national Swedish study. Osteoporos Int 32:343–352
Excellence. NIfHaC (2019) NICE guideline [NG132]: Hyperparathyroidism (primary): diagnosis, assessment and initial management. https://www.nice.org.uk/guidance/ng132/chapter/Recommendations#diagnostic-testing-in-primary-care
Acknowledgements
NOGG are grateful to our external peer reviewers, stakeholders, and the Committee of Scientific Advisors of the International Osteoporosis Foundation and by the European Society for the Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases for their endorsement of this manuscript.
Funding
National Institute for Health Research,CS-2018–18-ST2-010,Zoe Paskins
No funding source/body was involved in the development and/or writing of this guideline, all members gave up their time voluntarily, without any remuneration for their work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The National Osteoporosis Guideline Group (NOGG) Guideline Writing Group is divided into two groups, a Guideline Development Group (GDG) and an Expert Advisory Group (EAG). The GDG consists of 8 voting members (CLG, JC, JE, JK, RL, SL, ZP, JT), and 3 lay members (JB, JP, NV). One member has a financial disclosure, unrelated to drug development (JC received honoraria for speaking at a CME approved bone academy meeting and for consultancy relating to the characterisation of people at very high fracture risk), the remaining 10 have no financial disclosures. ZP is funded by the National Institute for Health Research (NIHR) (Clinician Scientist Award (CS-2018–18-ST2-010)/NIHR Academy). ZP has undertaken consultancy for non-promotional activities for UCB and waived any rights to any fees. One member (JK) is also a member of the FRAX group, hence all FRAX-based recommendations had to be agreed by consensus of all the GDG. The Chair (CLG) has no disclosures. The EAG is composed of 9 members, all of whom have relevant conflicts of interest. DA has received advisory/speaking fees from UCB and Internis Pharma and has shares in GSK. CC has received advisory/speaking fees from Amgen, Danone, Eli Lilly, GSK, Kyowa Kirin, Medtronic, Merck, Nestle, Novartis, Pfizer, Roche, Servier, Shire, Takeda, and UCB. NG is on the advisory board of UCB, Novo Nordisk, Shire/Takeda and has received fees from UCB and Takeda. NCH has received advisory board/consultancy fees/honoraria from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, UCB, Radius, Servier, Shire, Consilient Healthcare, and Internis Pharma. EMC has received advisory/honoraria from Amgen, Consilient Healthcare, Kyowa Kirin, Eli Lilly, Fresenius Kabi, Internis, ObsEva, Radius Inc and Redx Pharma and received research funding from Amgen, Internis, and Radius Inc. KM has received conference sponsorship from Abbvie, UCB, Novartis, Kyowa Kirin, Lilly, Pfizer, and Internis, and advisory/honoraria from Alexion. KP received advisory/speaker fees from UCB which were then donated to charities. DR has received advisory fees from UCB and Osteolabs. MS has received advisory/speaker fees from UCB, and speaker fees from Amgen and Gedeon Richter. All members of the GDG and EAG have read NICE’s ‘Policy on declaring and managing interests for NICE advisory boards’ (version 1.1, July 2019). Disclosures of all members of both groups are available on the NOGG website. Both the GDG and the EAG attend online meetings and take part in email discussions. However, voting on the recommendations is restricted to the GDG. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jean Bowden, Jane Parker, and Nic Vine are public and patient representatives.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gregson, C.L., Armstrong, D.J., Bowden, J. et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 17, 58 (2022). https://doi.org/10.1007/s11657-022-01061-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-022-01061-5