Abstract
Objective
To test the hypothesis that β -glucan enhances protective qi (PQi), an important Chinese medicine (CM) concept which stipulates that a protective force circulates throughout the body surface and works as the first line of defense against “external pernicious influences”.
Methods
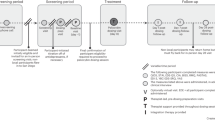
A total of 138 participants with PQi deficiency (PQD) were randomized to receive β -glucan (200 mg daily) or placebo for 12 weeks. Participants’ PQi status was assessed every 2 weeks via conventional diagnosis and a standardized protocol from which a PQD severity and risk score was derived. Indices of participants’ immune and general health status were also monitored, including upper respiratory tract infection (URTI), saliva secretory IgA (sIgA), and self-reported measures of physical and mental health (PROMIS).
Results
PQi status was not significantly different between the β -glucan and placebo treatment groups at baseline but improved significantly in the β -glucan (vs. placebo) group in a time-dependent manner. The intergroup differences [95% confidence interval (CI)] in severity score (scale: 1–5), risk score (scale: 0–1), and proportion of PQD participants (%) at finish line was 0.49 (0.35–0.62), 0.48 (0.35–0.61), and 0.36 (0.25–0.47), respectively. Additionally, β -glucan improved URTI symptom (scale: 1–9) and PROMIS physical (scale: 16.2–67.7) and mental (scale: 21.2–67.6) scores by a magnitude (95% CI) of 1.0 (0.21–1.86), 5.7 (2.33–9.07), and 3.0 (20.37–6.37), respectively, over placebo.
Conclusions
β -glucan ameliorates PQi in PQD individuals. By using stringent evidence-based methodologies, our study demonstrated that Western medicine-derived remedies, such as β -glucan, can be employed to advance CM therapeutics. (ClinicalTrial.Gov registry: NCT03782974)
Similar content being viewed by others
Data availability: Data are available upon request.
References
Chan G, Chan W, Sze D. The effects of β -glucan on human immune and cancer cells. J Hematol Oncol 2009;2:25.
Bashir KMI, Choi JS. Clinical and physiological perspectives of β -glucans: the past, present, and future. Int J Mol Sci 2017;18:9.
Levy M, Wu J, Shi J, Cheng H, Ou X, Bernstein I, et al. A proof-of-concept and feasibility study to evaluate the effect of β -glucan on protective qi deficiency in adults. Chin J Integr Med 2020 [Epub ahead of print].
Wu J, Cheng H, Shi J, Yin W, Wang J, Ou X, et al. Development and validation of a diagnostic risk score for assessing a TCM condition, protective qi deficiency, in adults. Eur J Integr Med 2020;35:101097.
Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials 1988;9:345–364.
Zhu F, Du B, Xu B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll 2016;25:275–288.
Talbott S, Talbott J. Effect of BETA 1, 3/1, 6 GLUCAN on upper respiratory tract infection symptoms and mood state in marathon athletes. J Sport Sci Med 2009;8:509–515.
Talbott SM, Talbott JA. Baker’s yeast beta-glucan Supplement reduces upper respiratory symptoms and improves mood state in stressed women. J Am Coll Nutr 2012;31:295–300.
Bergendiova K, Tibenska E, Majtan J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur J Appl Physiol 2011;111: 2033–2040.
Babineau TJ, Hackford A, Kenler A, Bistrian B, Forse RA, Fairchild PG, et al. A phase II multicenter, double-blind, randomized, placebocontrolled study of three dosages of an immunomodulator (PGG-Glucan) in high-risk surgical patients. Arch Surg 1994;129:1204–1210.
Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res 2009;18:873–880.
Keller SD, Yang M, Treadwell MJ, Werner EM, Hassell KL. Patient reports of health outcome for adults living with sickle cell disease: Development and testing of the ASCQ-Me item banks, Health Qual Life Outcomes 2014;12:125.
National Institute of Health. Screeners at a glance. Available at: https://dietassessmentprimer.cancer.gov/profiles/screeners/.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67:1–48.
Wobbrock JO, Findlater L, Gergle D, Higgins JJ. The aligned rank transform for nonparametric factorial analyses using only anova procedures, in: Proc. 2011 Annu. Conf. Hum. Factors Comput. Syst. -CHI’ 11, ACM Press, New York, New York, USA, 2011: 143. Available at: https://doi.org/10.1145/1978942.1978963.
Koo TK, M.Y. Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;2:155–163.
Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393.
Volman JJ, Ramakers JD, Plat J. Dietary modulation of immune function by β -glucans. Physiol Behav 2008;94:276–284.
McFarlin BK, Carpenter KC, Davidson T, McFarlin MA. Baker’s yeast beta glucan supplementation increases salivary IgA and decreases cold/flu symptomatic days after intense exercise. J Diet 2013;10(Suppl):171–183.
Bergendiova K, Tibenska E, Majtan J. Pleuran (β -glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur J Appl Physiol 2011;111:2033–2040.
Lehne G, Haneberg B, Gaustad P, Johansen PW, Preus H, Abrahamsen TG. Oral administration of a new soluble branched β -1,3-D-glucan is well tolerated and can lead to increased salivary concentrations of immunoglobulin A in healthy volunteers. Clin Exp Immunol 2006;143:65–69.
Richter J, Svozil V, Král V, Dobiásová LR, Vetvicka V. β-glucan affects mucosal immunity in children with chronic respiratory problems under physical stress: clinical trials. Ann Transl Med 2015;3:52.
McFarlin BK, Carpenter KC, Davidson T, McFarlin MA. Baker’s yeast beta glucan supplementation increases salivary IgA and decreases cold/flu symptomatic days after intense exercise. J Diet Suppl 2013;10:171–183.
Evans M, Falcone PH, Crowley DC, Sulley AM, Campbell M, Zakaria N, et al. Effect of a euglena gracilis fermentate on immune function in healthy, active adults: a randomized, double-blind, placebo-controlled trial. Nutrients 2019;11:2926.
Kahn SE, Cooper ME, Del Prato S, Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014;383:1068–1083.
Boh B, Berovic M, Zhang J, Lin ZB. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol Annu Rev 2007;13:265–301.
China Pharmacopeia Committe. China Pharmacopeia. 1st ed. Beijing: China Medical Science and Technology Press; 2015.
Author information
Authors and Affiliations
Contributions
Wu JR, Cheng HJ, Shi JP, Yin WD, Wang J, Levy M, Sinnott R, and Tian JQ designed the research (project conception and development of overall research plan); Wu JR, Shi JP, Yin WD, Wang J, Ou XQ, Chen JL, and Bernstein I were on-site investigators who conducted the research (hands-on conduct of the trial and data collection); Cheng HJ, Tian JQ, and Levy M processed the data and performed statistical analysis; Levy M and Cheng HJ wrote the Institutional Research Board (IRB) proposal and registered the study at clinicaltrial. gov.; Maddela R participated in study design and oversight of the trial progress; Tian JQ, Levy M, and Cheng HJ are the persons who primarily wrote the manuscript; Maddela R edited the manuscript; Wu JR and Cheng HJ contributed equally to this work; Tian JQ had primary responsibility for final content.
Corresponding author
Ethics declarations
Cheng HJ, Levy M, Maddela R, Sinnott R, and Tian JQ were employees of USANA Health Science, the manufacturer of Proglucamune® at the time of the study. Wu JR, Cheng HJ, Shi JP, Yin WD, Wang J, Ou XQ, and Chen JL were product distributors of USANA Health Science at the time of the study.
Additional information
Supported by USANA Health Science Inc., the manufacturer of Proglucamune®
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wu, Jr., Cheng, Hj., Shi, Jp. et al. β -Glucan Improves Protective Qi Status in Adults with Protective Qi Deficiency—A Randomized, Placebo-Controlled, and Double-Blinded Trial. Chin. J. Integr. Med. 28, 394–402 (2022). https://doi.org/10.1007/s11655-021-3444-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-021-3444-0