Abstract
Objective
To investigate the effect and potential mechanisms of rutaecarpine (Rut) in a rat artery balloon-injury model.
Methods
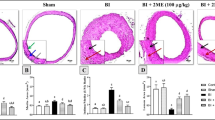
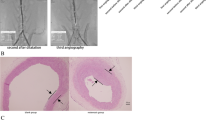
The intimal hyperplasia model was established by rubbing the endothelia with a balloon catheter in the common carotid artery (CCA) of rats. Fifty rats were randomly divided into five groups, ie. sham, model, Rut (25, 50 and 75 mg/kg) with 10 rats of each group. The rats were treated with or without Rut (25, 50, 75 mg/kg) by intragastric administration for 14 consecutive days following injury. The morphological changes of the intima were evaluated by hematoxylin-eosin staining. The expressions of proliferating cell nuclear antigen (PCNA) and smooth muscle (SM) α-actin in the ateries were assayed by immunohistochemical staining. The mRNA expressions of c-myc, extracellular signal-regulated kinase 2 (ERK2), MAPK phosphatase-1 (MKP-1) and endothelial nitric oxide synthase (eNOS) were determined by real-time reverse transcription-polymerase chain reaction. The protein expressions of MKP-1 and phosphorylated ERK2 (p-ERK2) were examined by Western blotting. The plasma contents of nitric oxide (NO) and cyclic guanosine 3',5'-monophosphate (cGMP) were also determined.
Results
Compared with the model group, Rut treatment significantly decreased intimal thickening and ameliorated endothelial injury (P<0.05 or P<0.01). The positive expression rate of PCNA was decreased, while the expression rate of SM α-actin obviously increased in the vascular wall after Rut (50 and 75 mg/kg) administration (P<0.05 or P<0.01). Furthermore, the mRNA expressions of c-myc, ERK2 and PCNA were downregulated while the expressions of eNOS and MKP-1 were upregulated (P<0.05 or P<0.01). The protein expressions of MKP-1 and the phosphorylation of ERK2 were upregulated and downregulated after Rut (50 and 75 mg/kg) administration (P<0.05 or P<0.01), respectively. In addition, Rut dramatically reversed balloon injury-induced decrease of NO and cGMP in the plasma (P<0.05 or P<0.01).
Conclusion
Rut could inhibit the balloon injury-induced carotid intimal hyperplasia in rats, possibly mediated by promotion of NO production and inhibiting ERK2 signal transduction pathways.
Similar content being viewed by others
References
Dangas G, Fuster V. Management of restenosis after coronary intervention. Am Heart J 1996;132:428–436.
Thiel WH, Esposito CL, Dickey DD, Dassie JP, Long ME, Adam J, et al. Smooth muscle cell-targeted RNA aptamer inhibits neointimal formation. Mol Ther 2016;24:779–787.
Wu X, Liu W, Jiang H, Chen J, Wang J, Zhu R, et al. Kindlin-2 siRNA inhibits vascular smooth muscle cell proliferation, migration and intimal hyperplasia via Wnt signaling. Int J Mol Med 2016;37:436–444.
Yu PJ, Ferrari G, Pirelli L, Gulkarov I, Galloway AC, Mignatti P, et al. Vascular injury and modulation of MAPKs: a targeted approach to therapy of restenosis. Cell Signal 2007;19:1359–1371.
Li Y, McRobb LS, Khachigian LM. Inhibition of intimal thickening after vascular injury with a cocktail of vascular endothelial growth factor and cyclic Arg-Gly-Asp peptide. Int J Cardiol 2016;220:185–191.
Janssens S, Flaherty D, Nong Z, Varenne O, van Pelt N, Haustermans C, et al. Human endothelial nitric oxide synthase gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation after balloon injury in rats. Circulation 1998;97:1274–1281.
Jia S, Hu C. Pharmacological effects of rutaecarpine as a cardiovascular protective agent. Molecules 2010;15:1873–1881.
Sheu JR, Hung WC, Lee YM, Yen MH. Mechanism of inhibition of platelet aggregation by rutaecarpine, an alkaloid isolated from Evodia rutaecarpa. Eur J Pharmacol 1996;318:469–475.
Li D, Peng J, Xin HY, Luo D, Zhang YS, Zhou Z, et al. Calcitonin gene-related peptide-mediated antihypertensive and anti-platelet effects by rutaecarpine in spontaneously hypertensive rats. Peptides 2008;29:1781–1788.
Sheu JR, Hung WC, Wu CH, Lee YM, Yen MH. Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa, on platelet plug formation in in vivo experiments. Br J Haematol 2000;110:110–115.
Hu CP, Xiao L, Deng HW, Li YJ. The cardioprotection of rutaecarpine is mediated by endogenous calcitonin relatedgene peptide through activation of vanilloid receptors in guinea-pig hearts. Planta Med 2002;68:705–709.
Yi HH, Rang WQ, Deng PY, Hu CP, Liu GZ, Tan GS, et al. Protective effects of rutaecarpine in cardiac anaphylactic injury is mediated by CGRP. Planta Med 2004;70:1135–1139.
Li JZ, Peng J, Xiao L, Zhang YS, Liao MC, Li XH, et al. Reversal of isoprenaline-induced cardiac remodeling by rutaecarpine via stimulation of calcitonin gene-related peptide production. Can J Physiol Pharmacol 2010;88:949–959.
Wang GJ, Wu XC, Chen CF, Lin LC, Huang YT, Shan J, et al. Vasorelaxing action of rutaecarpine: effects of rutaecarpine on calcium channel activities in vascular endothelial and smooth muscle cells. J Pharmacol Exp Ther 1999;289:1237–1244.
Xu Y, Hou HH, Li Q, Zhang JY, Sun AS. Comparison of inhibitory effects of Wu Zhuyu alkaloids on proliferation of vascular smooth muscle cells in rat. Lishizhen Med Mater Res (Chin) 2013;24:2079–2080.
Li YJ, Zhang F, Gong QH, Wu Q, Yu LM, Sun AS. Rutaecarpine inhibits angiotensin II-induced proliferation in rat vascular smooth muscle cells. Chin J Integr Med 2014:20:682–687.
Gao Y, Deng J, Yu XF, Yang DL, Gong QH, Huang XN. Ginsenoside Rg1 inhibits vascular intimal hyperplasia in balloon-injured rat carotid artery by down-regulation of extracellular signal-regulated kinase 2. J Ethnopharmacol 2011;18;138:472–478.
Xu Y, Hou HH, Li Q, Zhang JY, Sun AS. Comparison of the anti-proliferative effect of alkaloids isolated from Wu-Zhu-Yu in rat vascular smooth muscle cells. Lishizhen Med Mater Res (Chin) 2013;24:2079–2080.
Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell 2007;129:665–679.
Iatropoulos MJ, Williams GM. Proliferation markers. Exp Toxicol Pathol 1996;48:175–181.
Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 1995;75:487–517.
Wang L, Zheng J, Du Y, Huang Y, Li J, Liu B, et al. Cartilage oligomeric matrix protein maintains the contractile phenotype of vascular smooth muscle cells by interacting with alpha(7)beta(1) integrin. Circ Res 2010;106:514–525.
Pei C, Qin S, Wang M, Zhang S. Regulatory mechanism of human vascular smooth muscle cell phenotypic transformation induced by NELIN. Mol Med Rep 2015;12:7310–7316.
Koyama H, Bornfeldt KE, Fukumoto S, Nishizawa Y. Molecular pathways of cyclic nucleotide-induced inhibition of arterial smooth muscle cell proliferation. J Cell Physiol 2001;186:1–10.
O'Connor DM, O'Brien T. Nitric oxide synthase gene therapy: progress and prospects. Expert Opin Biol Ther 2009;9:867–878.
Hogg ME, Varu VN, Vavra AK, Popowich DA, Banerjee MN, MartinezJ, et al. Effect of nitric oxide on neointimal hyperplasia based on sex and hormone status. Free Radic Biol Med 2011;50:1065–1074.
Shapiro PS, Ahn NG. Feedback regulation of raf-1 and mitogen-activated protein (MAP) kinase kinases 1 and 2 by map kinase phosphatase-1 (MKP-1). J Biol Chem 1998;273:1788–1793.
Lai K, Wang H, Lee WS, Jain MK, Lee ME, Haber E, et a1. Mitogen-activated protein kinase phosphatase-1 in rat arterial smooth muscle cell proliferation. J Clin Invest 1996;98:1560–1567.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Y., Chen, Xp., Zhang, F. et al. Rutaecarpine Inhibits Intimal Hyperplasia in A Balloon-Injured Rat Artery Model. Chin. J. Integr. Med. 24, 429–435 (2018). https://doi.org/10.1007/s11655-017-2900-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-017-2900-3