Abstract
Objectives
To explore the phytotherapeutic-activities of Moringa oleifera (MO) seeds on painful diabetic neuropathy in alloxan-induced diabetic mice.
Methods
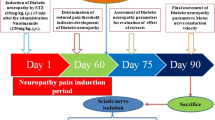
The bio-guided fractionation of MO utilizing column chromatography aided with GC-MS was used to detect the most active constituent of MO. Hyperalgesia, using tail-flick and hot-plate latency experiments, and mechanical-allodynia, utilizing von-Frey filaments, were evaluated before and after 8 weeks of intraperitoneal alloxan administration (180 mg/kg). Serum catalase and insulin levels, body weight and blood glucose levels (BGL), alpha-glucosidase inhibition, lipid peroxidation and glycated hemoglobin (HbA1c) were measured to evaluate both alloxan-induced diabetes mellitus and diabetic painful neuropathy (DPN).
Results
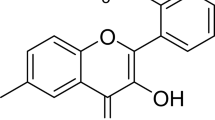
Beta-sitosterol (BSL) was proved to be the most active constituent of MO. The administration of MO (40, 60 and 80 mg/kg) or BSL (18, 25 and 35 mg/kg) significantly attenuated hyperalgesia and tactile allodynia (P⩽0.05), compared with tramadol (10 mg/kg) acting as a positive control, in alloxan-treated animals (n=7 per group). Moreover, MO and BSL have improved insulin secretion, in vivo antioxidant catalase, lipid peroxidation, acute and subchronic BGL, and normalized alpha-glucosidase and HbA1c levels.
Conclusions
The observed insulin secretagogue, alpha-glucosidase inhibition, hypoglycemic and antioxidant potentials might be responsible for MO and BSL antinociception and neuroprotective mechanism. MO and BSL have shown good glycemic-control and powerful neuroprotective properties which might serve as potential lead-compounds for further analysis.
Similar content being viewed by others
References
Spadiene A, Savickiene N, Ivanauskas L, Jakstas V, Skesters A, Silova A, Rodovicius H. Antioxidant effects of Camellia sinensis L. extract in patients with type 2 diabetes. J Food Drug Analysis 2014;22:505–511.
WHO. Global guideline for type 2 diabetes. Diabetes Res Clin Pract 2014;104:1–52.
Elosta A, Ghous T, Ahmed N. Natural products as antiglycation agents: possible therapeutic potential for diabetic complications. Curr Diabetes Rev 2012;8:92–108.
Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a fl avonoid antioxidant, prevents and protects streptozotocininduced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res 2005;51:117–123.
Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962.
Adam SH, Giribabu N, Kassim N, Kumar KE, Brahmayya M, Arya A, et al. Protective effect of aqueous seed extract of Vitis Vinifera against oxidative stress, infl ammation and apoptosis in the pancreas of adult male rats with diabetes mellitus. Biomed Pharmacother 2016;81:439–452.
Raafat KM, Jassar H, Aboul-Ela M, El-Lakany A. Protective effects of Origanum majorana L. against neurodegeneration: fingerprinting, isolation and in vivo glycine receptors behavioral model. Int J Phytomed 2013;5:46–57.
Casoli T, Spazzafumo L, Di Stefano G, Conti F. Role of diffuse low-level heteroplasmy of mitochondrial DNA in Alzheimer’s disease neurodegeneration. Front Aging Neurosci 2015;7:142.
Raafat K, Aboul-Ela M, El-Lakany A. Alloxan-induced diabetic thermal hyperalgesia, prophylaxis and phytotherapeutic effects of Rheum ribes L. in mouse model. Arch Pharm Res 2014:1–10.
Zhou J, Du X, Long M, Zhang Z, Zhou S, Zhou J, et al. Neuroprotective effect of berberine is mediated by MAPK signaling pathway in experimental diabetic neuropathy in rats. Eur J Pharmacol 2016;774:87–94.
da Costa AV, Calábria LK, Furtado FB, de Gouveia NM, Oliveira RJdS, de Oliveira VN, et al. Neuroprotective effects of Pouteria ramiflora (Mart.) Radlk (Sapotaceae) extract on the brains of rats with streptozotocin-induced diabetes. Metabol Brain Dis 2013;28:411–419.
Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia 2012;83:650–659.
Shanmugam KR, Mallikarjuna K, Kesireddy N, Sathyavelu Reddy K. Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol 2011;49:893–897.
Zhu D, Wang L, Zhou Q, Yan S, Li Z, Sheng J, Zhang W. (+)-Catechin ameliorates diabetic nephropathy by trapping methylglyoxal in type 2 diabetic mice. Mol Nutr Food Res 2014;58:2249–2260.
Raafat K, Wurglics M, Schubert-Zsilavecz M. Prunella vulgaris L. active components and their hypoglycemic and antinociceptive effects in alloxan-induced diabetic mice. Biomed Pharmacother 2016;84:1008–1018.
Jaiswal D, Rai PK, Mehta S, Chatterji S, Shukla S, Rai DK, et al. Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac J Trop Med 2013;6:426–432.
Chuang PH, Lee CW, Chou JY, Murugan M, Shieh BJ, Chen HM. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour Technol 2007;98:232–236.
Biswas S, Chowdhury A, Das J, Roy A, Zahid M. Pharmacological potentials of Moringa oleifera Lam.: a review. Int J Pharm Sci Res 2012;3:305–310.
Tie J, Jiang M, Li H, Zhang S, Zhang X. A comparison between Moringa oleifera seed presscake extract and polyaluminum chloride in the removal of direct black 19 from synthetic wastewater. Industr Crops Prod 2015;74:530–534.
Duong S, Strobel N, Buddhadasa S, Stockham K, Auldist M, Wales B, et al. Rapid measurement of phytosterols in fortified food using gas chromatography with flame ionization detection. Food Chem 2016;211:570–576.
Jimenez-Suarez V, Nieto-Camacho A, Jimenez-Estrada M, Alvarado Sanchez B. Anti-infl ammatory, free radical scavenging and alpha-glucosidase inhibitory activities of Hamelia patens and its chemical constituents. Pharm Biol 2016:1–9.
Pandey A, Negi PS. Traditional uses, phytochemistry and pharmacological properties of Neolamarckia cadamba: a review. J Ethnopharmacol 2016;181:118–135.
Saeidnia S, Ara L, Hajimehdipoor H, Read RW, Arshadi S, Nikan M. Chemical constituents of Swertia longifolia Boiss. with alpha-amylase inhibitory activity. Res Pharm Sci 2016;11:23–32.
Raafat K, Breitinger U, Mahran L, Ayoub N, Breitinger H-G. Synergistic inhibition of glycinergic transmission in vitro and in vivo by flavonoids and strychnine. Toxicol Sci 2010;118:171–182.
Kwon YI, Apostolidis E, Shetty K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour Technol 2008;99:2981–2988.
Sarmento B, Ribeiro A, Veiga F, Ferreira D. Development and validation of a rapid reversed-phase HPLC method for the determination of insulin from nanoparticulate systems. Biomed Chromatogr 2006;20:898–903.
Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, et al. Mouse models of diabetic neuropathy. Neurobiol Dis 2007;28:276–285.
Ulugol A, Oltulu C, Gunduz O, Citak C, Carrara R, Shaqaqi MR, et al. 5-HT7 receptor activation attenuates thermal hyperalgesia in streptozocin-induced diabetic mice. Pharmacol Biochem Behav 2012;102:344–348.
Fox A, Gentry C, Patel S, Kesingland A, Bevan S. Comparative activity of the anti-convulsants oxcarbazepine, carbamazepine, lamotrigine and gabapentin in a model of neuropathic pain in the rat and guinea-pig. Pain 2003;105:355–362.
Yasmineh WG, Kaur TP, Blazar BR, Theologides A. Serum catalase as marker of graft-vs-host disease in allogeneic bone marrow transplant recipients: pilot study. Clin Chem 1995;41:1574–1580.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–358.
Raafat K, El-Lakany A. Acute and subchronic in-vivo effects of Ferula hermonis L. and Sambucus nigra L. and their potential active isolates in a diabetic mouse model of neuropathic pain. BMC Complement Altern Med 2015;15:257.
Chandiran IS, Jayaveera KN, Karimulla S. Preliminary phytochemical and preclinical toxicity studies of Grewia serrulata DC. Drug Invent Today 2013;5:267–274.
Diallo A, Eklu-Gadegbeku K, Amegbor K, Agbonon A, Aklikokou K, Creppy E, et al. In vivo and in vitro toxicological evaluation of the hydroalcoholic leaf extract of Ageratum conyzoides L. (Asteraceae). J Ethnopharmacol 2014;155:1214–1218.
World Health Organization. WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues. Geneva: World Health Organization; 2007.
da Silva AR, Moreira Lda R, Brum Eda S, de Freitas ML, Boligon AA, Athayde ML, et al. Biochemical and hematological effects of acute and sub-acute administration to ethyl acetate fraction from the stem bark Scutia buxifolia Reissek in mice. J Ethnopharmacol 2014;153:908–916.
Obrosova IG. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics 2009;6:638–647.
Abo KA, Fred-Jaiyesimi AA, Jaiyesimi AE. Ethnobotanical studies of medicinal plants used in the management of diabetes mellitus in South Western Nigeria. J Ethnopharmacol 2008;115:67–71.
Shim YJ, Doo HK, Ahn SY, Kim YS, Seong JK, Park IS, et al. Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J Ethnopharmacol 2003;85:283–287.
Ranilla LG, Kwon YI, Apostolidis E, Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour Technol 2010;101:4676–4689.
Torrance GW. Utility approach to measuring health-related quality of life. J Chronic Dis 1987;40:593–603.
Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain sev. Pain sev. Pain sev. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage 2005;30:374–385.
Wolford ST, Schroer RA, Gohs FX, Gallo PP, Brodeck M, Falk HB, Ruhren R. Referenc. Referenc. Referenc. Reference range data base for serum chemistry and hematology values in laboratory animals. J Toxicol Environ Health 1986;18:161–188.
Acknowledgment
Authors would like to thank Mrs. G. Onsy (BAU, Lebanon) for proof-reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
11655_2017_2758_MOESM1_ESM.pdf
Neuroprotective effects of Moringa oleifera: Bio-guided GC-MS identification of active compounds in diabetic neuropathic pain model
Rights and permissions
About this article
Cite this article
Raafat, K., Hdaib, F. Neuroprotective effects of Moringa oleifera: Bio-guided GC-MS identification of active compounds in diabetic neuropathic pain model. Chin. J. Integr. Med. (2017). https://doi.org/10.1007/s11655-017-2758-4
Received:
Published:
DOI: https://doi.org/10.1007/s11655-017-2758-4