Abstract
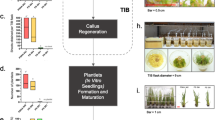
Conservation of Saccharum spp. germplasm as ex situ collections of plants has a high cost, and in natural conditions, the plants remain exposed to pests, pathogens, and natural disasters. Long-term preservation of plant germplasm is important for agricultural biodiversity and food safety, so the aim of this study was to develop a cryogenic procedure for cryopreservation of sugarcane germplasm. The first study compared droplet vitrification and encapsulation-vitrification techniques for cryopreservation of in vitro shoot tips of Saccharum spp. variety Halaii. The best regeneration rate (70.9%) was obtained from 45-min PVS2 vitrification solution-treated shoot tips via the droplet vitrification technique. This technique was tested on two other Saccharum sp. varieties, and the best regeneration rates for varieties NG 57-024 and H 83-6179 were 63.3 and 76.3%, respectively. Shoots derived from cryopreserved shoot tip buds developed well-formed roots, and were easily acclimated to greenhouse conditions. The second study evaluated genetic stability of the cryopreserved varieties using ten inter-simple sequence repeat primers. A total of 211 (Halaii), 198 (H83-6179), and 201 (NG 57-024) reproducible bands, ranging from 125 to 5500 bp, were scored with this technique. One hundred genetic stability was detected from Halaii and H 83-6179 whereas 98.5% genetic stability was detected from varieties of NG 57-024. The PCR reactions showed that there was no crucial variation on genetic stability for all cryopreserved varieties.





Similar content being viewed by others
References
Al-Ababneh SS, Karam NS, Shibli RA (2002) Cryopreservation of sour orange (Citrus aurantium L.) shoot tips. In Vitro Cell Dev Biol Plant 38:602–607
Barraco G, Sylvestre I, Engelmann F (2011) Comparing encapsulation-dehydration and droplet-vitrification for cryopreservation of sugarcane (Saccharum spp.) shoot tips. Sci Hortic 130:320–324
Benson EE (1999) Cryopreservation. In: Benson EE (ed) Plant conservation biotechnology. Taylor and Francis, London, pp 83–95
Berding N, Roach BT (1987) Sugarcane improvement through breeding. In: Heinz DJ (ed) Developments in crop science. Elsevier, Amsterdam, pp 143–210
Charoensub R, Hirai D, Sakai A (2004) Cryopreservation of in vitro-grown shoot tips of cassava by encapsulation-vitrification method. CryoLetters 25:51–58
Choudhary R, Chaudhury R, Malik SK, Kumar S, Pal D (2013) Genetic stability of mulberry germplasm after cryopreservation by two-step freezing technique. Afr J Biotechnol 12:5983–5993
Dereuddre J (1992) Cryopreservation of in vitro cultures of plant cells and organs by vitrification and dehydration. In: Dattee Y, Dumas C, Gallais A (eds) Reproductive biology and plant breeding. Springer, Berlin, pp 291–300
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Focus 12:13–15
Engelmann F (1991) In vitro conservation of tropical plant germplasm—a review. Euphytica 57:227–243
Engelmann F (2004) Plant cryopreservation: progress and prospects. In Vitro Cell Dev Biol Plant 40:427–433
Gamez-Pastrana R, Martinez-Ocampo Y, Beristain CI, GonzalezArnao MT (2004) An improved cryopreservation protocol for pineapple apices using encapsulation-vitrification. Cryo Letters 25:405–414
Gonzalez Arnao MT, Engelmann F, Huet C, Urra C (1993) Cryopreservation of encapsulated apices of sugarcane: effect of freezing procedure and histology. CryoLetters 14:303–308
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. CryoLetters 25:3–22
Hirai D (2001) Studies on cryopreservation of vegetatively propagated crops by encapsulation vitrification method. Rep Hokkaido Pref Agric Exp Sta 99:1–58
Hirai D, Sakai A (2003) Simplified cryopreservation of sweet potato [Ipomoea batatas (L.) Lam.] by optimizing conditions for osmoprotection. Plant Cell Rep 21:961–966
Kaya E (2015) ISSR analysis for determination of genetic diversity and relationship in some Turkish olive (Olea europaea L) cultivars. Not Bot Horti Agrobot Cluj Napoca 43:96–99
Kaya E, Alves A, Rodrigues L, Jenderek M, Hernandez-Ellis M, Ozudogru A, Ellis D (2013) Cryopreservation of Eucalyptus genetic resources. CryoLetters 34:608–618
Kaya E, Souza F, Yilmaz-Gokdogan E, Ceylan M, Jenderek M (2017) Cryopreservation of citrus seed via dehydration followed by immersion in liquid nitrogen. Turk J Biol 41:242–248
Kocsis JJ, Harkaway S, Santoyo MC, Snyder R (1968) Dimethyl sulfoxide: interactions with aromatic hydrocarbons. Science 160:427–428
Lambardi M, Sharma KK, Thorpe TA (1993) Optimization of in vitro bud induction and plantlet formation from mature embryos of Aleppo pine (Pinus halepensis Mill.) In Vitro Cell Dev Biol Plant 29:189–199
Leunufna S, Keller ERJ (2003) Investigating a new cryopreservation protocol for yams (Dioscorea spp.) Plant Cell Rep 21:1159–1166
Marascuilo LA, McSweeney M (1977) Non-parametric and distribution free methods for the social sciences. In: Marascuilo LA (ed) McSweeney M. Books/Cole Publication, Belmont, pp 141–147
Martin C, González I, Kremer C, González-Benito ME (2014) Cryopreservation and genetic stability of shoot cultures in vegetatively propagated species: mint and chrysanthemum. Acta Hortic 1039:127–132
Martins-Lopes P, Lima-Brito J, Gomes S, Meirinhos J, Santos L, Guedes-Pinto H (2007) RAPD and ISSR molecular markers in Olea europaea L.: genetic variability and molecular cultivar identification. Genet Res Crop Evol 54:117–128
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ozudogru EA, Kaya E (2012) Cryopreservation of Thymus cariensis and T. vulgaris shoot tips: comparison of three vitrification-based methods. CryoLetters 33:363–375
Ozudogru EA, Kaya E, Kirdok E, Issever-Ozturk S (2011) In vitro propagation from young and mature explants of thyme (Thymus vulgaris and T. longicaulis) resulting in genetically stable shoots. In Vitro Cell Dev Biol Plant 47:309–320
Panis B, Piette B, Swennen R (2005) Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Sci 168:45–55
Panis B, Swennen R, Engelmann F (2001) Cryopreservation of plant germplasm. Acta Hort 560:79–86
Paul H, Daigny G, Sangwan-Norreel BS (2000) Cryopreservation of apple (Malus×domestica Borkh.) shoot tips following encapsulation-dehydration or encapsulation-vitrification. Plant Cell Rep 19:768–774
Paunesca A (2009) Biotechnology for endangered plant conservation: a critical overview. Rom Biotech Letters 14:4095–4104
Rao NK (2004) Plant genetic resources: advancing conservation and use through biotechnology. Afr J Biotechnol 3:136–145
Reddy MP, Sarla N, Siddiq EA (2002) Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica 128:9–17
Rafique T, Yamamoto S, Fukui K, Mahmood Z, Niino T (2015) Cryopreservation of sugarcane using the V cryo-plate technique. CryoLetters 36:51–59
Rohlf FJ (1998) NTSYS-pc numerical taxonomy and multivariate analysis system version 2. 0. Exeter publications, New York, USA, pp 1–44
Saha S, Adhikari S, Dey T, Ghosh P (2016) RAPD and ISSR based evaluation of genetic stability of micropropagated plantlets of Morus alba L. variety S-1. Meta Gene 7:7–15
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLetters 28:151–172
Sakai A, Matsumato T, Hirai D, Charoensub R (2002) Survival of tropical apices cooled to −196°C by vitrification. In: Li PH, Palva ET (eds) Plant cold hardiness, gene regulation and genetic engineering. Kluwer Academic/Plenum Publishers, New York, pp 109–119
Shibli RA, Al-Juboory KH (2000) Cryopreservation of ‘Nabali’ olive (Olea europea L.) somatic embryos by encapsulation-dehydration and encapsulation-vitrification. CryoLetters 21:357–366
Souza FVD, Kaya E, de Jesus VL, de Souza EH, de Oliveira Amorim VB, Skogerboe D, Matsumoto T, Alves AAC, da Silva Ledo CA, Jenderek MM (2016) Droplet-vitrification and morphohistological studies of cryopreserved shoot tips of cultivated and wild pineapple genotypes. Plant Cell Tissue Organ Cult 124:351–360
Tannoury M, Ralambosoa J, Kaminsky M, Dereuddre J (1991) Cryopreservation by vitrification of alginate-coated carnation (Dianthus caryophyllus L.) cultured in vitro plantlets. C. R. Acad. Sci. Paris, pp 633–638
Towill LE, Bonnart R (2003) Cracking in a vitrification solution during cooling or warming does not effect growth of cryopreserved mint shoot tips. CryoLetters 24:341–346
Valles MP, Wang ZY, Montavon P, Potrykus I, Spangenberg G (1993) Analysis of genetic stability of plants regenerated trom suspension cultures and protoplasts of meadow rescue (Festuca pratensis Huds.) Plant Cell Rep 12:101–106
Vijayan K (2005) Inter simple sequence repeat (ISSR) polymorphism and its application in mulberry genome analysis. Int J Indust Entomol 10:79–86
Acknowledgements
This work was supported by Mugla Sitki Kocman University, Scientific Research Projects Coordination Unit (Mugla, Turkey, MSKU-BAP 16/021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Barbara Reed
Rights and permissions
About this article
Cite this article
Kaya, E., Souza, F.V.D. Comparison of two PVS2-based procedures for cryopreservation of commercial sugarcane (Saccharum spp.) germplasm and confirmation of genetic stability after cryopreservation using ISSR markers. In Vitro Cell.Dev.Biol.-Plant 53, 410–417 (2017). https://doi.org/10.1007/s11627-017-9837-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-017-9837-2