Abstract
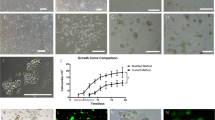
The objective of the study is to evaluate efficiency of in vitro isolation and myogenic differentiation of mesenchymal stem cells (MSCs) derived from adipose connective tissue (AD-MSCs), bone marrow (BM-MSCs), and skeletal muscle tissue (MC-MSCs). MSCs were isolated from adipose connective tissue, bone marrow, and skeletal muscle tissue of two adult 6-wk-old rats. Cultured MSCs were treated with 5-azacytidine (AZA) to induce myogenic differentiation. Isolated MSCs and differentiated cells were evaluated by immunocytochemistry (ICC), fluorescence-activated cell sorting (FACS), PCR, and RT-PCR. AD-MSCs showed the highest proliferation rate while BM-MSCs had the lowest one. In ICC, isolated MSCs had strong CD90- and CD44-positive expression and negative expression of CD45, CD31, and CD34, while AZA-treated MSCs had strong positive desmin expression. In FACS analysis, AD-MSCs had the highest percentage of CD90- and CD44-positive-expressing cells (99% and 96%) followed by BM-MSCs (97% and 94%) and MC-MSCs (92% and 91%).At 1 wk after incubation with AZA treatment, the peak of myogenin expression reached 93% in differentiated MC-MSCs, 83.3% in BM-MSCs, and 77% in AD-MSCs. MSCs isolated from adipose connective tissue, bone marrow, and skeletal muscle tissue have the same morphology and phenotype, but AD-MSCs were the most easily accessible and had the highest rate of growth on cultivation and the highest percentage of stem cell marker expression. Moreover, although MC-MSCs showed the highest rate of myogenic differentiation potential and expression of myoblast markers, AD-MSCs and BM-MSCs still can be valuable alternatives. The differentiated myoblastic cells could be an available new choice for myoblastic auto-transplantation in regeneration medicine.









Similar content being viewed by others
References
Adhim Z.; Matsuoka T.; Bito T.; Shigemura K.; Lee K. M.; Kawabata M.; Fujisawa M.; Nibu K.; Shirakawa T. In vitro and in vivo inhibitory effect of three Cox-2 inhibitors and epithelial-to-mesenchymal transition in human bladder cancer cell lines. Br. J. Cancer 105: 393–402; 2011.
Ahmed S. A.; Gogal Jr. R. M.; Walsh J. E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170: 211–224; 1994.
Asakura A.; Komaki M.; Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 68: 245–253; 2001.
Beauchamp J. R.; Heslop L.; Yu D. S.; Tajbakhsh S.; Kelly R. G.; Wernig A.; Buckingham M. E.; Partridge T. A.; Zammit P. S. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. The Journal of cell biology 151: 1221–1234; 2000.
Beier J. P.; Bitto F. F.; Lange C.; Klumpp D.; Arkudas A.; Bleiziffer O.; Boos A. M.; Horch R. E.; Kneser U. Myogenic differentiation of mesenchymal stem cells co-cultured with primary myoblasts. Cell biology international 35: 397–406; 2011.
Boonen K. J.; Post M. J. The muscle stem cell niche: regulation of satellite cells during regeneration. Tissue engineering 14: 419–431; 2008.
Caplan A. I. Mesenchymal stem cells. J. Orthop. Res. 9: 641–650; 1991.
Carlson M. E.; Conboy I. M. Loss of stem cell regenerative capacity within aged niches. Aging cell 6: 371–382; 2007.
Cossu G.; Mavilio F. Myogenic stem cells for the therapy of primary myopathies: wishful thinking or therapeutic perspective? J. Clin. Invest. 105: 1669–1674; 2000.
Curzer H. The ethics of embryonic stem cell research. The Journal of medicine and philosophy 29: 533–562; 2004.
De Bari C.; Dell’Accio F.; Luyten F. P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis and rheumatism 44: 85–95; 2001a.
De Bari C.; Dell’Accio F.; Tylzanowski P.; Luyten F. P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis and rheumatism 44: 1928–1942; 2001b.
Dominici M.; Le Blanc K.; Mueller I.; Slaper-Cortenbach I.; Marini F.; Krause D.; Deans R.; Keating A.; Prockop D.; Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317; 2006.
Dreyfus P. A.; Chretien F.; Chazaud B.; Kirova Y.; Caramelle P.; Garcia L.; Butler-Browne G.; Gherardi R. K. Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches. Am. J. Pathol. 164: 773–779; 2004.
Drost A. C.; Weng S.; Feil G.; Schafer J.; Baumann S.; Kanz L.; Sievert K. D.; Stenzl A.; Mohle R. In vitro myogenic differentiation of human bone marrow-derived mesenchymal stem cells as a potential treatment for urethral sphincter muscle repair. Ann. N. Y. Acad. Sci. 1176: 135–143; 2009.
Friedenstein A. J.; Petrakova K. V.; Kurolesova A. I.; Frolova G. P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6: 230–247; 1968.
Fu Q.; Song X. F.; Liao G. L.; Deng C. L.; Cui L. Myoblasts differentiated from adipose-derived stem cells to treat stress urinary incontinence. Urology 75: 718–723; 2010.
Gage FH (2000) Mammalian neural stem cells. Science 287: 1433–8
Gang E. J.; Jeong J. A.; Hong S. H.; Hwang S. H.; Kim S. W.; Yang I. H.; Ahn C.; Han H.; Kim H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem cells (Dayton, Ohio) 22: 617–624; 2004.
Gimble J. M. Adipose tissue-derived therapeutics. Expert. Opin. Biol. Ther. 3: 705–713; 2003.
Gimble J. M.; Katz A. J.; Bunnell B. A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 100: 1249–1260; 2007.
Hass R.; Kasper C.; Bohm S.; Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9: 12; 2011.
Kobe U (2011) Laws and Regulations of Animal experimentation at Kobe University: http://www.kobe-u.ac.jp/research/animal-experiments/laws-regulations.htm.
Lee R. H.; Kim B.; Choi I.; Kim H.; Choi H. S.; Suh K.; Bae Y. C.; Jung J. S. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell. Physiol. Biochem. 14: 311–324; 2004.
Liu Z. J.; Zhuge Y.; Velazquez O. C. Trafficking and differentiation of mesenchymal stem cells. J. Cell. Biochem. 106: 984–991; 2009.
Meirelles Lda S.; Nardi N. B. Methodology, biology and clinical applications of mesenchymal stem cells. Front. Biosci. 14: 4281–4298; 2009.
Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. Journal of Nihon Medical School = Nihon Ika Daigaku zasshi 76: 56–66; 2009.
Mollmann H.; Nef H. M.; Voss S.; Troidl C.; Willmer M.; Szardien S.; Rolf A.; Klement M.; Voswinckel R.; Kostin S.; Ghofrani H. A.; Hamm C. W.; Elsasser A. Stem cell-mediated natural tissue engineering. Journal of Cellular and Molecular Medicine 15: 52–62; 2011.
Musina R. A.; Bekchanova E. S.; Sukhikh G. T. Comparison of mesenchymal stem cells obtained from different human tissues. Bulletin of Experimental Biology and Medicine 139: 504–509; 2005.
Musina R. A.; Bekchanova E. S.; Belyavskii A. V.; Sukhikh G. T. Differentiation potential of mesenchymal stem cells of different origin. Bulletin of Experimental Biology and Medicine 141: 147–151; 2006.
Phinney D. G. Building a consensus regarding the nature and origin of mesenchymal stem cells. J Cell Biochem Suppl 38: 7–12; 2002.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–7
Rangwala S. M.; Lazar M. A. Transcriptional control of adipogenesis. Annu. Rev. Nutr. 20: 535–559; 2000.
Sakaguchi Y.; Sekiya I.; Yagishita K.; Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis and Rheumatism 52: 2521–2529; 2005.
Wakitani S.; Saito T.; Caplan A. I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 18: 1417–1426; 1995.
Wu G.; Zheng X.; Jiang Z.; Wang J.; Song Y. Induced differentiation of adipose-derived stromal cells into myoblasts. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao 30: 285–290; 2010.
Yamamoto N.; Akamatsu H.; Hasegawa S.; Yamada T.; Nakata S.; Ohkuma M.; Miyachi E.; Marunouchi T.; Matsunaga K. Isolation of multipotent stem cells from mouse adipose tissue. J. Dermatol. Sci. 48: 43–52; 2007.
Yoshimura H.; Muneta T.; Nimura A.; Yokoyama A.; Koga H.; Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell and tissue research 327: 449–462; 2007.
Zuk P. A.; Zhu M.; Ashjian P.; De Ugarte D. A.; Huang J. I.; Mizuno H.; Alfonso Z. C.; Fraser J. K.; Benhaim P.; Hedrick M. H. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell 13: 4279–4295; 2002.
Zuk P. A.; Zhu M.; Mizuno H.; Huang J.; Futrell J. W.; Katz A. J.; Benhaim P.; Lorenz H. P.; Hedrick M. H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue engineering 7: 211–228; 2001.
Grant support
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Meligy, F.Y., Shigemura, K., Behnsawy, H.M. et al. The efficiency of in vitro isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. In Vitro Cell.Dev.Biol.-Animal 48, 203–215 (2012). https://doi.org/10.1007/s11626-012-9488-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-012-9488-x