Abstract
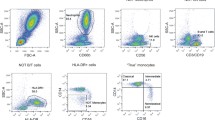
The p38α mitogen-activated protein kinase (MAPK) is essential in controlling the production of many proinflammtory cytokines, and its specific inhibitor can effectively block their production for treating human diseases. To effectively identify highly specific p38α inhibitors in vivo, we developed an ex vivo mouse blood cell-based assay by flow cytometry to measure the intracellular p38α kinase activation. We first attempted to identify the individual blood cell population in which the p38α kinase pathway is highly expressed and activated. Based on CD11b, combined with Ly-6G cell surface expression, we identified two distinct subsets of non-neutrophilic myeloid cells, CD11bMedLy-6G− and CD11bLoLy-6G−, and characterized them as monocytes and natural killer (NK) cells, respectively. Then, we demonstrated that only monocytes, not NK cells, expressed a high level of p38α kinase, which was rapidly activated by anisomycin stimulation as evidenced by the phosphorylation of both p38 and its substrate, MAPKAP-K2 (MK2). Finally, the p38α kinase pathway activation in monocytes was fully inhibited by a highly selective p38α kinase inhibitor dose-dependently in vitro and in vivo. In conclusion, we demonstrated an effective method for separating blood monocytes from other cells and for detecting the expression level and activation of the p38α kinase pathway in monocytes, which provided a new approach for the rapid identification of specific p38α inhibitors.





Similar content being viewed by others
References
Anderson K. L.; Smith K. A.; Perkin H.; Hermanson G.; Anderson C. G.; Jolly D. J.; Ma R. A.; Torbett B. E. PU.1 and the granulocyte- and macrophage colony-stimulating factor receptors play distinct roles in late-stage myeloid cell differentiation. Blood 94:2310–2318; 1999.
Andersson K.; Sundler R. Signalling to translational activation of tumour necrosis factor-alpha expression in human THP-1 cells. Cytokine 12:1784–1787; 2000.
Andreakos E. Targeting cytokines in autoimmunity: new approaches, new promise. Expert Opin Biol Ther 3:435–447; 2003.
Burke B.; P.ridmore A.; Harraghy N.; Collick A.; Brown J.; Mitchell T. Transgenic mice showing inflammation-inducible overexpression of granulocyte macrophage colony-stimulating factor. Clin Diagn Lab Immunol 11:588–598; 2004.
Comalada M.; Xaus J.; Valledor A. F.; Lopez-Lopez C.; Pennington D. J.; Celada A. PKC epsilon is involved in JNK activation that mediates LPS-induced TNF-alpha, which induces apoptosis in macrophages. Am J Physiol Cell Physiol 285:C1235–C1245; 2003.
de Dios A.; Shih C., et al. Design of potent and selective 2-aminobenzimidazole-based p38a MAP kinase inhibitors with extracellular in vitro efficacy. J Med Chem 48:2270–2273; 2005.
Di Santo J. P.; Vosshenrich C. A. Bone marrow versus thymic pathways of natural killer cell development. Immunol Rev 214:35–46; 2006 (Review).
Geissmann F.; Jung S.; Littman D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82; 2003.
Grage-Grieben E.; Flad H. D.; Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol 69:11–20; 2001 (Review).
Henderson R. B.; Hobbs J. R.; Mathies M.; Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood 102:328–335; 2003.
House D.; Chinh N. T.; Hien T. T.; Parry C. P.; Ly N. T.; Diep T. S.; Wain J.; Dunstan S.; White N. J.; Dougan G.; Farrar J. J. Cytokine release by lipopolysaccharide-stimulated whole blood from patients with typhoid fever. J Infect Dis. 186(2):240–245; 2002.
Jacquin C.; Gran D. E.; Lee S. K.; Lorenzo J. A.; Aguila H. L. Identification of multiple osteoclast precursor populations in murine bone marrow. J Bone Miner Res 21:67–77; 2006.
Kotlyarov A.; Gaestel M. Is MK2 (mitogen-activated protein kinase-activated protein kinase 2) the key for understanding post-transcriptional regulation of gene expression? Biochem Soc Trans 30:959–963. 2002 (Review).
Kotlyarov A.; Neininge A.; Schubert C.; Eckert R.; Birchmeier C.; Volk H. D.; Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol 1:94–97; 1999.
Lagasse E.; Weissman I. L. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods 197:139–150; 1996.
Lee R.; Dominguez Celia. MAP kinase p38 inhibitors: clinical results and an intimate look at their interactions with p38a protein. Current Med Chem 12:2979–2794 2005.
Lehner M. D.; Schwoebel F.; Kotlyarov A.; Leist M.; Gaestel M.; Hartung T. Mitogen-activated protein kinase-activated protein kinase 2-deficient mice show increased susceptibility to Listeria monocytogenes infection. J Immunol 168:4667–4673; 2002.
McCormick C.; Gan D. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 307:739–741; 2005.
Nagendra S.; Schlueter A. J. Absence of cross-reactivity between murine Ly-6C and Ly-6G. Cytometry A 58:195–200; 2004.
Neininger A.; Kontoyiannis D.; Kotlyarov A.; Winzen R.; Eckert R.; Volk H. D.; Holtmann H.; Kolli G.; Gaestel M.; MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J Biol Chem 277:3065–3068; 2002.
Nikolic T.; Bouma G.; Drexhage H. A.; Leenen P. J. Diabetes-prone NOD mice show an expanded subpopulation of mature circulating monocytes, which preferentially develop into macrophage-like cells in vitro. J Leukoc Biol 78:70–79; 2005.
Nockher W. A.; Wiemer J.; Scherberich J. E. Haemodialysis monocytopenia: differential sequestration kinetics of CD14+ CD16+ and CD14++ blood monocyte subsets. Clin Exp Immunol 123:49–55; 2001.
Peifer C.; Wagner G.; Laufer S. New approaches to the treatment of inflammatory disorders small molecule inhibitors of p38 MAP kinase. Curr Top Med Chem 6:113–149; 2006 (Review)
Saklatvala J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr Opin Pharmacol 4:372–377; 2004 (Review).
Schieven G. L. The biology of p38 kinase: a central role in inflammation. Curr Top Med Chem 5:921–928; 2005 (Review).
Strauss-Ayali D.; Conrad S. M.; Mosser, D. M. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol 82:244–252; 2007 (Review).
Sunderkotter C.; Nikolic T.; Dillon M. J.; Van Rooijen N.; Stehling M.; Drevets D. A.; Leenen P. J. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 172:4410–4417; 2004.
Weber C.; Belge K. U.; von Hundelshausen P.; Draude G.; Steppich B.; Mack M.; Frankenberger M.; Weber K. S.; Ziegler-Heitbrock H. W. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol 67:699–704; 2000.
Wiktor-Jedrzejczak W.; Gordon S. Cytokine regulation of the macrophage (M phi) system studied using the colony stimulating factor-1-deficient op/op mouse. Physiol Rev 76:927–947; 1996 (Review).
Acknowledgments
We would like to thank Deltagen for providing MAPKAPK2 knockout mice and Larry Mann for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
Rights and permissions
About this article
Cite this article
Zhao, J., Evans, G., Li, W. et al. Rapid and quantitative detection of p38 kinase pathway in mouse blood monocyte. In Vitro Cell.Dev.Biol.-Animal 44, 145–153 (2008). https://doi.org/10.1007/s11626-008-9088-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-008-9088-y