Abstract
Background
Polypharmacy and use of inappropriate medications have been linked to increased risk of falls, hospitalizations, cognitive impairment, and death. The primary objective of this review was to evaluate the effectiveness, comparative effectiveness, and harms of deprescribing interventions among community-dwelling older adults.
Methods
We searched OVID MEDLINE Embase, CINAHL, and the Cochrane Library from 1990 through February 2019 for controlled clinical trials comparing any deprescribing intervention to usual care or another intervention. Primary outcomes were all-cause mortality, hospitalizations, health-related quality of life, and falls. The secondary outcome was use of potentially inappropriate medications (PIMs). Interventions were categorized as comprehensive medication review, educational initiatives, and computerized decision support. Data abstracted by one investigator were verified by another. We used the Cochrane criteria to rate risk of bias for each study and the GRADE system to determine certainty of evidence (COE) for primary outcomes.
Results
Thirty-eight low and medium risk of bias clinical trials were included. Comprehensive medication review may have reduced all-cause mortality (OR 0.74, 95% CI: 0.58 to 0.95, I2 = 0, k = 12, low COE) but probably had little to no effect on falls, health-related quality of life, or hospitalizations (low to moderate COE). Nine of thirteen trials reported fewer PIMs in the intervention group. Educational interventions probably had little to no effect on all-cause mortality, hospitalizations, or health-related quality of life (low to moderate COE). The effect on falls was uncertain (very low COE). All 11 education trials that included PIMs reported fewer in the intervention than in the control groups. Two of 4 computerized decision support trials reported fewer PIMs in the intervention arms; none included any primary outcomes.
Discussion
In community-dwelling people aged 65 years and older, medication deprescribing interventions may provide small reductions in mortality and use of potentially inappropriate medications.
Registry Information
PROSPERO - CRD42019132420.
Similar content being viewed by others
INTRODUCTION
More than 40% of people in the USA aged ≥65 years take 5 or more prescription medications on a regular basis to control or prevent disease symptoms and complications.1 Exposure to multiple medications, known as polypharmacy, is associated with increased risk of undesirable outcomes, such as falls, cognitive impairment and other geriatric syndromes, hospitalizations, and death.2,3 The number of medications a person is taking may be the single most important predictor of adverse drug effects.3 Furthermore, approximately half of older adults take one or more potentially inappropriate medications (PIMs), which includes duplicative medications and those with known risks or without a clear indication.4
Efforts have been underway for more than 30 years to develop and test interventions to mitigate the adverse effects of polypharmacy and inappropriate medication use. Initially, drug discontinuation efforts were focused on stopping specific medications considered to be problematic in older adults.5,6 This has evolved into a more holistic approach, called “deprescribing,” that considers medications in the context of the individual’s comorbidities, functional status, treatment goals, and life expectancy.1,5 Deprescribing has been defined as “the clinically supervised process of stopping or reducing the dose of medications when they cause harm or no longer provide benefit.”1,5,7
Several systematic reviews on deprescribing have been recently published.8,9 However, to our knowledge, none has focused on the comparative effectiveness of different deprescribing interventions for community-dwelling older adults. This gap in the literature may be an obstacle to implementation of deprescribing initiatives within clinics and healthcare systems.10 We conducted this systematic review to determine the effectiveness, comparative effectiveness, and harms of deprescribing interventions in community-dwelling older adults.
METHODS
This manuscript is based on a Department of Veterans Affairs (VA) Evidence-based Synthesis Program report prepared for the VA Center for Medication Safety in Aging and available at https://www.hsrd.research.va.gov/publications/esp/. The purpose of the report, which was also supported by the VA Pharmacy Benefits Management and the Geriatrics and Extended Care Services, was to inform implementation of deprescribing within VA. These services collaborate within VA to identify deprescribing best practices to improve the health of Veterans.
Data Sources
We searched MEDLINE from 1990 to February 2019 using Medical Subject Headings (MeSH) and key words for deprescribing, medication therapy management, decision support systems, geriatric assessment, electronic health records, medical order systems, polypharmacy, aged population, and Veterans (Appendix A). We searched Embase, the Cumulative Index of Nursing and Allied Health (CINAHL), and the Cochrane Library using search strategies based on the MEDLINE strategy. Citations were entered into DistillerSR (Evidence Partners). The full search strategy is available in Appendix A.
Study Selection
We included randomized and cluster randomized controlled trials and controlled clinical trials that evaluated any deprescribing intervention for community-dwelling adults aged ≥ 65 years and reported one or more of our outcomes of interest. We categorized deprescribing interventions based on their dominant component: comprehensive medication review (CMR), education and feedback, or computerized decision support. Two investigators independently evaluated each abstract, which moved to full-text review if either reviewer considered the citation eligible. At full-text review, agreement of 2 reviewers was required for study inclusion. Disputes were resolved by discussion with input from a third reviewer, if needed. We excluded articles not published in English.
Data Abstraction and Quality Assessment
Data were abstracted by one investigator or research associate and verified by a second. Abstracted data included study design, inclusion and exclusion criteria, description of intervention and control arms, subject characteristics (e.g., age, gender, race/ethnicity, comorbidities, physical and cognitive status), select laboratory values, and baseline number of medications. Our a priori primary outcomes were quality of life, all-cause mortality, hospitalizations, falls, adverse drug withdrawal events, major adverse cardiac events, and delirium; none of the included studies reported the latter 3 outcomes. In addition, we report potentially inappropriate medications (PIMs), the most commonly reported medication outcome in the included studies. Polypharmacy has been defined in many ways;11 in this review, we accepted authors’ definitions.
Each study’s risk of bias was rated by one co-investigator or research associate and verified by a second. Overall risk of bias for a study was rated as low, medium, or high based on the Cochrane risk of bias criteria for randomized trials and cluster randomized trials: sequence generation, allocation concealment, recruitment bias, baseline imbalance, blinded outcome assessment, incomplete cluster data, incomplete outcome data, and selective outcome reporting.12 High risk of bias studies were excluded from the analyses.
Data Synthesis and Analysis
We pooled results if studies were deemed low or medium risk of bias and outcome measures, populations, interventions, and study designs were comparable. Data were analyzed in Comprehensive Meta-Analysis version 3 (Biostat). Categorical outcomes data were pooled using the Peto odds ratio (Peto OR) method or risk ratios (RR) with corresponding 95% confidence intervals (CIs). Magnitude of statistical heterogeneity was assessed with the I2 statistic (I2 > 75% may indicate substantial heterogeneity).13 Standardized mean differences (SMDs) between the intervention and control groups, with corresponding 95% CIs, were calculated for continuous efficacy outcomes and were interpreted by applying Cohen’s definition of small (0.2), medium (0.5), and large (0.8) effects.14 For studies reporting categorical outcomes that were not pooled due to differences in study design or outcome definition, we calculated absolute effects (risk differences) with corresponding 95% CIs for individual trials. Cluster randomized controlled trials were not pooled with randomized controlled trials if they did not report adjustment for clustering.
Certainty of evidence, our confidence in the estimates of effect, for primary efficacy outcomes was rated using GRADE software (GRADEpro GDT: GRADEpro Guideline Development Tool [Software] McMaster University and Evidence Prime, Inc. 2015. Ontario, Canada). Certainty of evidence was graded for each outcome as high, moderate, low, or very low by evaluating 4 critical domains (risk of bias, consistency, directness, precision). High certainty indicates high confidence that the estimate of effect reflects the true effect while very low certainty indicates that evidence is either unavailable or does not permit a conclusion. Discrepancies in certainty of evidence ratings were resolved by discussion with final determination arrived through consensus.
Role of the Funding Source
The funding source (Department of Veterans Affairs, Office of Research and Development, Health Services Research and Development Service) assigned the topic and reviewed the protocol but was not involved in data collection, analysis, manuscript preparation, or submission.
RESULTS
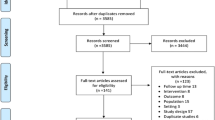
As shown in Figure 1, 44 of the 278 full-text articles reviewed for eligibility met inclusion criteria.15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67 Six were rated high risk of bias and are not included in the analyses.62,63,64,65,66,67 Of the remaining 38, 12 were randomized controlled trials and 26 were cluster randomized controlled trials. Included studies were similar with respect to study population (older adults taking multiple medications and living in the community) and setting (outpatient primary care clinics). Most interventions focused on general deprescribing, although some studies targeted medication classes (e.g., psychotherapeutic) or specific goals (e.g., falls reduction). We report results by the type of intervention studied: CMR (k = 22), educational interventions (k = 12), or computerized decision support (k = 4). For more detailed information on the included studies, see Appendix B.
Comprehensive Medication Review
Twenty-two trials evaluated the effect of comprehensive medication review (CMR) compared with a control group, most often usual care.15,16,17,19,20,25,27,28,29,31,32,33,34,35,36,37,38,39,41,42,43,44,45,54,58,59,60,61 Generally the CMR interventions were conducted by a pharmacist (k = 16) and included a chart review, in-person patient interview, and provider consultation, culminating in recommendations for medication regimen changes. Eight studies also included a follow-up intervention with patients to reinforce the recommendations, such as 1 to 3 home care visits by nurses or telephone calls by pharmacists over the 2- to 12-month follow-up period. Five trials were conducted in the USA, one in Canada, one in Malaysia, and 15 in Europe. We judged the risk of bias to be low in 5 trials and medium in 17. Studies enrolled 9482 patients, with study sample sizes ranging from 25 to 1403. Certainty of evidence for primary outcomes is summarized in Table 1.
All-Cause Mortality
All-cause mortality was reported in 12 trials enrolling 4875 patients with follow-up ranging from 1 to 12 months.15,16,17,19,20,27,28,29,36,37,38,39,44,45,59,61 Compared with usual care, CMR resulted in a 26% relative risk reduction (OR 0.74, 95% CI: 0.58 to 0.95, I2 = 0) corresponding to a 1.4 percentage point absolute reduction (95% CI: − 2.7 to − 0.1) in all-cause mortality (Fig. 2). Overall, CMR may result in a small reduction in all-cause mortality (low certainty).
Hospitalizations
Hospitalizations over a wide range of follow-up durations were reported in 12 studies with a combined enrollment of 5672 participants.19,20,27,28,29,31,32,33,35,36,39,43,44,58,59,61 None of these studies reported a difference between the intervention and control groups with respect to the number of participants with one or more hospitalizations during follow-up. In the 6 RCTs that could be pooled,19,20,39,44,58,59,61 20.4% of people in the deprescribing group were hospitalized vs 19.8% in usual care for an absolute risk difference of 0.6% (95% CI: − 2.3 to 3.5) over follow-up durations ranging from 3 to 24 months (Fig. 3). Overall CMR probably results in little to no reduction in hospitalizations (moderate certainty).
Health-Related Quality of Life
Health-related quality of life was reported in 11 studies measured with either the EuroQual Quality of Life scale (EQ-5D or EQ-5D VAS) (k = 5), the Short Form Health Survey (SF-12/36 or physical, mental subscales) (k = 5), or both (k = 1).16,17,19,20,28,29,31,32,33,34,35,39,42,43,45,59 Nine studies reported no difference between the intervention and control groups in health-related quality of life at study end. Overall, CMR may result in little to no improvement in health-related quality of life scores (low certainty).
Falls
Four trials reported fall outcomes,16,17,41,59,60 only one of which found a difference between the intervention and control groups. This study (medium risk of bias) enrolled 620 adults ≥ 70 years old and focused on medications that might increase risk of falls. The intervention group had a 62% decrease in fall-related diagnoses during the 1-year study (OR 0.38, P < .01, CI not reported) despite no difference between groups in the total number of medications or number of psychoactive medications at follow-up.60
The IMPROveFALL trial (medium risk of bias) enrolled 612 older adults who had visited an emergency room because of a fall; there was no difference between the intervention and control groups at 12 months in the number of falls (37% vs 34%; absolute risk difference 4%, 95% CI: − 4 to 12).16,17 The other 2 trials did not designate falls as a primary outcome, had small sample sizes (N = 259 and 157), and had short lengths of follow-up (less than 3 months).41,59 Overall, CMR may result in little to no reduction in falls (low certainty).
Reduction in Potentially Inappropriate Medications
Nine of thirteen trials that reported a potentially inappropriate medication outcome found fewer PIMs in the intervention group than in the control group; the difference was statistically significant in 7 studies (Table 3).16,17,25,28,29,34,41,42,54 The variability in outcome definitions precluded pooling of results. However, we calculated standardized mean differences for the five trials that reported the Medication Appropriateness Index.15,28,29,34,42,43 In these trials, the intervention effect, as measured by Cohen’s d, was less than small in two,15,43 small in one,34 and moderate in two28,29,42 (see Appendix C).
Educational Interventions
We identified 12 trials18,21,22,23,24,30,40,46,47,50,51,52,53,55,57 that evaluated the effect of various educational interventions: provider education with feedback (k = 5); provider education without feedback (k = 2); patient education (k = 3); patient and provider education (k = 1); or patient and provider education with provider feedback (k = 1). The control groups were assigned either usual care (k = 10) or a sham intervention (i.e., targeting drugs that were not of interest, k = 2). Two trials were conducted in the USA, 3 in Canada, 6 in Europe, and 1 in Australia. We judged the risk of bias to be low in 6 trials and medium in the other 6. Outcomes were reported on a total of 3463 patients in the 9 smaller trials and on 252,684 in the 3 larger trials. Certainty of evidence for each primary outcome is summarized in Table 2.
All-Cause Mortality
All-cause mortality was reported in 6 trials (n = 121,314).18,21,22,23,24,30,40,53 None of the trials reported a difference between the intervention and control groups, and the data were not suitable for pooling due to heterogeneity of study interventions. Overall, educational interventions probably had no effect on all-cause mortality (moderate certainty).
Hospitalizations
Four of the 5 trials that reported hospitalizations found no difference between the intervention and control groups.21,22,23,24,40,53,57 Overall, education interventions probably do not reduce hospitalizations (moderate certainty).
Health-Related Quality of Life
Health-related quality of life was reported in 4 trials, none of which found a difference between the intervention and control groups.21,22,23,24,47,52 Overall, education interventions may have little to no effect on HRQoL (low certainty).
Falls
Two medium risk of bias trials reported falls and came to opposite conclusions. A US trial that randomized 169 people aged ≥ 65 at high risk for hospitalization or functional decline to enrollment in a chronic care clinic program or usual care. There was no difference in incidence of falls at 12 months between the 2 groups (43.5% vs 35.6%; P = .35).24 The second was an Australian trial that randomized 22 general practitioners to a provider education with feedback intervention or usual care.47 The physicians recruited 849 patients aged ≥ 65. At 12 months, the intervention group had lower odds of falls (OR: 0.61, 95% CI: 0.41 to 0.91; 20% vs 30%), injury (OR: 0.56, 95% CI: 0.32 to 0.96, 10% vs 18%), and injury requiring medical attention (OR: 0.46, 95% CI 0.30 to 0.70; 6% vs 13%). Overall, the effect of educational interventions on the risk of falls is not known (very low certainty).
Potentially Inappropriate Medications
All 11 trials that reported potentially inappropriate medications (PIMs) reported fewer PIMs in the intervention group than in the control group; the difference was statistically significant in 7 studies (Table 3).18,21,22,23,24,30,40,46,47,50,51,53,55,57
Computerized Decision Support
We identified 4 trials that evaluated the effect of computerized decision support.26,48,49,56 The interventions in these trials generally included electronic medical record alerts to pharmacists or providers identifying a PIM, sometimes with additional features such as a recommendation for a substitute medication. Two trials were conducted in the USA and 2 in Canada. Samples sizes ranged from 128 to 59,680 patients and study periods from 90 days to 13 months. All were considered medium risk of bias. In all 4 trials, reduction in PIMs was the only outcome reported (Table 3). Two reported a significant reduction of PIMs in the intervention group compared with the control group49,56 and 2 reported no intervention effect.26,48
DISCUSSION
We conducted this systematic review to evaluate the efficacy of deprescribing interventions in community-dwelling persons aged 65 or older. Study interventions included comprehensive medication review, provider and/or patient education, or computerized decision support. We found that deprescribing based on comprehensive medication review may reduce mortality and that all 3 types of interventions may reduce the number of PIMs. The evidence did not indicate that deprescribing either reduced or increased falls, hospitalizations, or health-related quality of life.
Although, to our knowledge, this is the first SR of controlled clinical trials that specifically addresses the comparative effectiveness of different deprescribing interventions in community-dwelling older adults, our findings are generally consistent with other recent reviews. A 2018 Cochrane review focused on older adults taking four or more medications in the community, hospital, or nursing home setting. The review, which included RCTs and non-randomized trials, controlled before and after studies and interrupted time series, concluded that it “was uncertain whether the interventions reduced the number of PIMs …and likely led to little or no difference in QOL or hospital admissions.”8 A systematic review of 116 experimental and observational studies of deprescribing interventions in older adults in any setting reported that compared with the control conditions, deprescribing reduced the number of inappropriate medications (mean difference − 0.49, 95% CI: − 0.7 to − 0.28, k = 3, N = 839), but had no demonstrable effect on quality of life or falls risk.9
Exploratory analysis of our data suggested some hypotheses that might be tested in future research. First, comprehensive medication reviews may reduce healthcare costs in addition to the reduction in mortality and PIMs reported here (see http://www.hsrd.research.va.gov/publications/esp/). Furthermore, follow-up interventions, such as phone calls or clinic visits with patients, may improve the effectiveness of comprehensive medication reviews. Second, provider education-only interventions are not effective. However, direct-to-consumer patient engagement programs with targeted educational material may be an efficient mechanism for reducing use of specific potentially inappropriate medications on a large scale. From the small group of computerized decision support trials, we were unable to identify any insights into which intervention components were most promising.
We acknowledge several limitations of this review. First, we included only English language publications. Second, the possibility of selective reporting and publication bias may have affected our findings. Third, this review was limited to the community setting; the effects of deprescribing for people in hospitals or long-term care facilities may differ. Fourth conclusions from the review are constrained by limitations of the available data, including the absence of data on major adverse cardiac events, adverse drug withdrawal events, and delirium and lack of standardized definitions of PIMs and other outcomes of interest. Finally, we identified no trials comparing two or more different active interventions with each other. Despite the evidence that polypharmacy is associated with increased risk of undesirable outcomes, such as falls, cognitive impairment, hospitalizations, medication burden, and costs among older adults, deprescribing has not been widely embraced.2 This may reflect patients’ concerns about the impact of medication discontinuation on their health and on their relationship with the prescribing providers, and providers’ concerns about meeting quality metrics, increased workload, patient acceptance, and overturning the prescribing decisions of colleagues.68 It might also reflect the lack of comparative effectiveness trials that would inform implementation efforts. Recent efforts to develop and disseminate evidence-based guidelines may facilitate broader adoption of deprescribing programs in healthcare systems.69
In conclusion, comprehensive medication review may result in a reduction in mortality and use of PIMs. Educational initiatives may reduce use of PIMs but have uncertain effects on quality of life and rates of hospitalizations and falls. Computer decision support interventions may reduce PIMs but have not reported clinical outcomes. We did not identify any studies that assessed the comparative effectiveness of the different deprescribing approaches. Future research should include well-designed comparative effectiveness trials conducted in a variety of settings, employing a uniform set of outcome measures and including process evaluations to guide subsequent implementation of effective interventions.
References
Linsky A, Gellad WF, Linder JA, Friedberg MW. Advancing the science of deprescribing: a novel comprehensive conceptual framework. J Am Geriatr Soc. 2019;67(10):2018-2022.
Mangin D, Bahat G, Golomb BA, et al. International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP): position statement and 10 recommendations for action. Drugs Aging. 2018;35(7):575-587.
Scott IA, Anderson K, Freeman CR, Stowasser DA. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201(7):390-392.
Reeve E, Bell JS, Hilmer SN. Barriers to optimising prescribing and deprescribing in older adults with dementia: a narrative review. Curr Clin Pharmacol. 2015;10(3):168-177.
Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. Review of deprescribing processes and development of an evidence-based, patient-centered deprescribing process. Br J Clin Pharmacol. 2014;78:738-747.
Page A, Clifford R, Potter K, Etherton-Beer C. A concept analysis of deprescribing medicaitons in older people. J Pharm Pract Res. 2018;48:132-148.
Thompson W, Farrell B. Deprescribing: what is it and what does the evidence tell us? Can J Hosp Pharm. 2013;66:201.
Rankin A, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database of Systematic Reviews. 2018;(9):CD008165.
Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: A systematic review and meta-analysis. BrJ Clin Pharmacol. 2016;82:583-623.
Thillainadesan J, Gnjidic D, Green S, Hilmer SN. Impact of deprescribing interventions in older hospitalised patients on prescribing and clinical outcomes: a systematic review of rnadomised trials. Drugs Aging. 2018;35:303-319.
Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011;Available from http://handbook.cochrane.org. (accessed July 23, 2019)
Higgins JP Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560.
Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale: Lawrence Earlbaum Associates. 1988.
Allard J, Hebert R, Rioux M, Asselin J, Voyer L. Efficacy of a clinical medication review on the number of potentially inappropriate prescriptions prescribed for community-dwelling elderly people. CMAJ. 2001;164(9):1291-1296.
Boyé NDA, Van der Velde N, De Vries OJ, et al. Effectiveness of medication withdrawal in older fallers: results from the Improving Medication Prescribing to reduce Risk Of FALLs (IMPROveFALL) trial. Age Ageing. 2017;46(1):142-146.
Polinder S, Boyé NDA, Mattace-Raso FUS, et al. Cost-utility of medication withdrawal in older fallers: results from the improving medication prescribing to reduce risk of FALLs (IMPROveFALL) trial. BMC Geriatr. 2016;16:179.
Bregnhoj L, Thirstrup S, Kristensen MB, Bjerrum L, Sonne J. Combined intervention programme reduces inappropriate prescribing in elderly patients exposed to polypharmacy in primary care. Eur J Clin Pharmacol. 2009;65(2):199-207.
Campins L, Serra-Prat M, Gozalo I, et al. Randomized controlled trial of an intervention to improve drug appropriateness in community-dwelling polymedicated elderly people. Fam Pract. 2017;34(1):36-42.
Campins L, Serra-Prat M, Palomera E, Bolibar I, Martinez MA, Gallo P. Reduction of pharmaceutical expenditure by a drug appropriateness intervention in polymedicated elderly subjects in Catalonia (Spain). Gac Sanit. 2019;33(2):106-111.
Clyne B, Smith SM, Hughes CM, et al. Effectiveness of a multifaceted intervention for potentially inappropriate prescribing in older patients in primary care: a cluster-randomized controlled trial (OPTI-SCRIPT Study). Ann Fam Med. 2015;13(6):545-553.
Clyne B, Smith SM, Hughes CM, Boland F, Cooper JA, Fahey T. Sustained effectiveness of a multifaceted intervention to reduce potentially inappropriate prescribing in older patients in primary care (OPTI-SCRIPT study). Implement Sci. 2016;11(1):79.
Gillespie P, Clyne B, Raymakers A, Fahey T, Hughes CM, Smith SM. Reducing potentially inappropriate prescribing for older people in primary care: cost-effectiveness of the OPTI-SCRIPT intervention. Int J Technol Assess Health Care. 2017;33(4):494-503.
Coleman EA, Grothaus LC, Sandhu N, Wagner EH. Chronic care clinics: a randomized controlled trial of a new model of primary care for frail older adults. J Am Geriatr Soc. 1999;47(7):775-783.
Denneboom W, Dautzenberg MG, Grol R, De Smet PA. Treatment reviews of older people on polypharmacy in primary care: cluster controlled trial comparing two approaches. Br J Gen Pract. 2007;57(542):723-731.
Fried TR, Niehoff KM, Street RL, et al. Effect of the Tool to Reduce Inappropriate Medications on medication communication and deprescribing. J Am Geriatr Soc. 2017;65(10):2265-2271.
Haag JD, Davis AZ, Hoel RW, et al. Impact of pharmacist-provided medication therapy management on healthcare quality and utilization in recently discharged elderly patients. Am Health Drug Benefits. 2016;9(5):259-267.
Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428-437.
Schmader KE, Hanlon JT, Landsman PB, Samsa GP, Lewis IK, Weinberger M. Inappropriate prescribing and health outcomes in elderly veteran outpatients. Ann Pharmacother. 1997;31(5):529-533.
Jager C, Freund T, Steinhauser J, et al. Impact of a tailored program on the implementation of evidence-based recommendations for multimorbid patients with polypharmacy in primary care practices-results of a cluster-randomized controlled trial. Implement Sci. 2017;12(1):8.
Jodar-Sanchez F, Malet-Larrea A, Martin JJ, et al. Cost-utility analysis of a medication review with follow-up service for older adults with polypharmacy in community pharmacies in Spain: the conSIGUE program. PharmacoEconomics. 2015;33(6):599-610.
Malet-Larrea A, Goyenechea E, Garcia-Cardenas V, et al. The impact of a medication review with follow-up service on hospital admissions in aged polypharmacy patients. Br J Clin Pharmacol. 2016:82:831-838.
Malet-Larrea A, Goyenechea E, Gastelurrutia MA, et al. Cost analysis and cost-benefit analysis of a medication review with follow-up service in aged polypharmacy patients. Eur J Health Econ. 2017;18(9):1069-1078.
Köberlein-Neu J, Mennemann H, Hamacher S, et al. Interprofessional medication management in patients with multiple morbidities. Dtsch Arztebl Int. 2016;113(44):741-748.
Krska J, Cromarty JA, Arris F, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing. 2001;30(3):205-211.
Kwint HF, Faber A, Gussekloo J, Bouvy ML. Effects of medication review on drug-related problems in patients using automated drug-dispensing systems: A pragmatic randomized controlled study. Drugs Aging. 2011;28(4):305-314.
Lampela P, Hartikainen S, Lavikainen P, Sulkava R, Huupponen R. Effects of medication assessment as part of a comprehensive geriatric assessment on drug use over a 1-year period: a population-based intervention study. Drugs Aging. 2010;27(6):507-521.
Rikala M, Korhonen MJ, Sulkava R, Hartikainen S. The effects of medication assessment on psychotropic drug use in the community-dwelling elderly. Int Psychogeriatr. 2011;23(3):473-484.
Lenaghan E, Holland R, Brooks A. Home-based medication review in a high risk elderly population in primary care--the POLYMED randomised controlled trial. Age Ageing. 2007;36(3):292-297.
Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898.
Meredith S, Feldman P, Frey D, et al. Improving medication use in newly admitted home healthcare patients: A randomized controlled trial. J Am Geriatr Soc. 2002;50(9):1484-1491.
Moga DC, Abner EL, Rigsby DN, et al. Optimizing medication appropriateness in older adults: a randomized clinical interventional trial to decrease anticholinergic burden. Alzheimers Res Ther. 2017;9(1):36.
Muth C, Uhlmann L, Haefeli WE, et al. Effectiveness of a complex intervention on Prioritising Multimedication in Multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial. BMJ Open. 2018;8(2):e017740.
Olesen C, Harbig P, Buus KM, Barat I, Damsgaard EM. Impact of pharmaceutical care on adherence, hospitalisations and mortality in elderly patients. Int J Clin Pharm. 2014;36(1):163-171.
Olsson IN, Runnamo R, Engfeldt P. Drug treatment in the elderly: an intervention in primary care to enhance prescription quality and quality of life. Scand J Prim Health Care. 2012;30(1):3-9.
Pimlott NJG, Hux JE, Wilson LM, Kahan M, Li C, Rosser WW. Educating physicians to reduce benzodiazepine use by elderly patients: a randomized controlled trial. CMAJ. 2003;168(7):835-839.
Pit SW, Byles JE, Henry DA, Holt L, Hansen V, Bowman DA. A quality use of medicines program for general practitioners and older people: a cluster randomised controlled trial. Med J Aust. 2007;187(1):23-30.
Price M, Davies I, Rusk R, Lesperance M, Weber J. Applying STOPP guidelines in primary care through electronic medical record decision support: randomized control trial highlighting the importance of data quality. JMIR Med Inform. 2017;5(2):e15.
Raebel MA, Charles J, Dugan J, et al. Randomized trial to improve prescribing safety in ambulatory elderly patients. J Am Geriatr Soc. 2007;55(7):977-985.
Rognstad S, Brekke M, Mdala I, Fetveit A, Gjelstad S, Straand J. Characteristics of GPs responding to an educational intervention to minimise inappropriate prescriptions: subgroup analyses of the Rx-PAD study. BJGP Open. 2018;2(1):bjgpopen18X101373.
Rognstad S, Brekke M, Fetveit A, Dalen I, Straand J. Prescription peer academic detailing to reduce inappropriate prescribing for older patients: a cluster randomised controlled trial. Br J Gen Pract. 2013;63(613):e554-562.
Schafer I, Kaduszkiewicz H, Mellert C, et al. Narrative medicine-based intervention in primary care to reduce polypharmacy: results from the cluster-randomised controlled trial MultiCare AGENDA. BMJ Open. 2018;8(1):e017653.
Schmidt-Mende K, Andersen M, Wettermark B, Hasselstrom J. Educational intervention on medication reviews aiming to reduce acute healthcare consumption in elderly patients with potentially inappropriate medicines-a pragmatic open-label cluster-randomized controlled trial in primary care. Pharmacoepidemiol Drug Saf. 2017;26(11):1347-1356.
Shim YW, Chua SS, Wong HC, Alwi S. Collaborative intervention between pharmacists and physicians on elderly patients: a randomized controlled trial. Ther Clin Risk Manag. 2018;14:1115-1125.
Simon SR, Smith DH, Feldstein AC, et al. Computerized prescribing alerts and group academic detailing to reduce the use of potentially inappropriate medications in older people. J Am Geriatr Soc. 2006;54(6):963-968.
Tamblyn R, Huang A, Perreault R, et al. The medical office of the 21st century (MOXXI): effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care. CMAJ. 2003;169(6):549-556.
Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Int Med. 2014;174(6):890-898.
Touchette DR, Masica AL, Dolor RJ, et al. Safety-focused medication therapy management: a randomized controlled trial. J Am Pharm Assoc. 2012;52(5):603-612.
Van Der Meer HG, Wouters H, Pont LG, Taxis K. Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: A randomised controlled trial. BMJ Open. 2018;8(7):e019042.
Weber V, White A, McIlvried R. An electronic medical record (EMR)-based intervention to reduce polypharmacy and falls in an ambulatory rural elderly population. J Gen Int Med. 2008;23(4):399-404.
Zermansky AG, Petty DR, Raynor DK, Freemantle N, Vail A, Lowe CJ. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ. 2001;323(7325):1340-1343.
Bryant LJM, Coster G, Gamble GD, McCormick RN. The General Practitioner-Pharmacist Collaboration (GPPC) study: a randomised controlled trial of clinical medication reviews in community pharmacy. Int J Pharm Practice. 2011;19(2):94-105.
Gnjidic D, Le Couteur DG, Abernethy DR, Hilmer SN. A pilot randomized clinical trial utilizing the drug burden index to reduce exposure to anticholinergic and sedative medications in older people. Ann Pharmacother. 2010;44(11):1725-1732.
Lenander C, Elfsson B, Danielsson B, Midlov P, Hasselstrom J. Effects of a pharmacist-led structured medication review in primary care on drug-related problems and hospital admission rates: a randomized controlled trial. Scand J Prim Health Care. 2014;32(4):180-186.
Richmond S, Morton V, Cross B, et al. Effectiveness of shared pharmaceutical care for older patients: RESPECT trial findings. Br J Gen Pract. 2010;60(570):14-20.
Steinman MA, Low M, Balicer RD, Shadmi E. Impact of a nurse-based intervention on medication outcomes in vulnerable older adults. BMC Geriatr. 2018;18(1):207.
Bernsten C, Bjorkman I, Caramona M, et al. Improving the well-being of elderly patients via community pharmacy-based provision of pharmaceutical care. A multicentre study in seven European countries. Drugs Aging. 2001;18(1):63-67.
Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
Farrell B, Conklin JU, Dolovich L, et al. Deprescribing guidelines: An international symposium on development, implementation, research and health professional education. Res Social Adm Pharm. 2019;15(6):780-789.
Funding
Funding to support the VA Evidence Synthesis Program site at the Minneapolis VA was provided by the Department of Veterans Affairs, Office of Research and Development, Health Services Research and Development Service.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Linsky was funded by grants from VA Health Services Research and Development (I21HX002406-01 and 5IK2HX001357-05). She is also a Co-Investigator for the Center for Medication Safety in Aging, a VA Patient Safety Center of Inquiry.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bloomfield, H.E., Greer, N., Linsky, A. et al. Deprescribing for Community-Dwelling Older Adults: a Systematic Review and Meta-analysis. J GEN INTERN MED 35, 3323–3332 (2020). https://doi.org/10.1007/s11606-020-06089-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-020-06089-2