Abstract
Purposes
The prognosis of localized extranodal natural killer/T-cell lymphoma, nasal type (ENKTL), has improved with the development of chemoradiotherapy. However, conventional extended-field radiotherapy may cause optic disorders. Our group has employed smaller radiation fields in an attempt to avoid toxicity. The efficacy and toxicity of treatments were evaluated.
Materials and methods
Chemoradiotherapy was delivered with a shrinking-field radiotherapy strategy. The endpoints of this study were overall survival (OS), local control (LC), progression-free survival (PFS), and toxicity.
Results
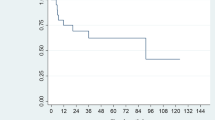
Fifteen patients with localized ENKTL were treated. After irradiation (median, 40 Gy) to the tumor plus a prophylactic volume, a reduced treatment volume to the tumor was boosted (median, 10 Gy). Twelve patients underwent chemoradiotherapy and 3 patients received radiotherapy alone. A complete response was achieved in 12 and a partial response in 3 patients. The 5-year OS, PFS, and LC rates were 80, 67, and 93 %, respectively. Distant recurrence occurred in 4 patients and locoregional and distant recurrence in 1 patient. Cataract (grade 3) and dry eye (grade 2) were observed as late adverse events in 1 patient each.
Conclusions
Sufficiently high OS and LC were achieved with acceptable toxicities. Appropriate target volumes may be smaller with newer chemotherapy regimens.



Similar content being viewed by others
References
Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–30.
Kwong YL. The diagnosis and management of extranodal NK/T-cell lymphoma, nasal-type and aggressive NK-cell leukemia. J Clin Exp Hematop. 2011;51:21–8.
Yamaguchi M. Current and future management of NK/T-cell lymphoma based on clinical trials. Int J Hematol. 2012;96:562–71.
Maeda E, Akahane M, Kiryu S, Kato N, Yoshikawa T, Hayashi N, et al. Spectrum of Epstein-Barr virus-related disease: a pictorial review. Jpn J Radiol. 2009;27:4–19.
Wang L, Wang ZH, Chen XQ, Li YJ, Wang KF, Xia YF, et al. First-line combination of gemcitabine, oxaliplatin, and l-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013;119:348–55.
Jiang M, Zhang H, Jiang Y, Yang Q, Xie L, Liu W, et al. Phase 2 trial of “sandwich” l-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer. 2012;118:3294–301.
Lin N, Song Y, Zheng W, Tu M, Xie Y, Wang X, et al. A prospective phase II study of l-asparaginase- CHOP plus radiation in newly diagnosed extranodal NK/T-cell lymphoma, nasal type. J Hematol Oncol. 2013;6:44.
Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2009;27:5594–600.
Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29:4410–6.
Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y, et al. Concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: an updated analysis of the Japan clinical oncology group study JCOG0211. J Clin Oncol. 2012;30:4044–6.
Isobe K, Uno T, Tamaru J, Kawakami H, Ueno N, Wakita H, et al. Extranodal natural killer/T-cell lymphoma, nasal type: the significance of radiotherapeutic parameters. Cancer. 2006;106:609–15.
Oh D, Ahn YC, Kim SJ, Kim WS, Ko YH. Concurrent chemoradiation therapy followed by consolidation chemotherapy for localized extranodal natural killer/T-cell lymphoma, nasal type. Int J Radiat Oncol Biol Phys. 2015;93:677–83.
Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh J, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27:6027–32.
The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–94.
Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24:612–8.
Shibamoto Y, Naruse A, Fukuma H, Ayakawa S, Sugie C, Tomita N. Influence of contrast materials on dose calculation in radiotherapy planning using computed tomography for tumors at various anatomical regions: a prospective study. Radiother Oncol. 2007;84:52–5.
Koom WS, Chung EJ, Yang WI, Shim SJ, Suh CO, Roh JK, et al. Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic viewpoints. Int J Radiat Oncol Biol Phys. 2004;59:1127–37.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–53.
Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45:1388–95.
Wilson WH. Treatment strategies for aggressive lymphomas: what works? Hematology Am Soc Hematol Educ Program. 2013;2013:584–90.
Yahalom J, Illidge T, Specht L, Hoppe RT, Li YX, Tsang R, et al. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:11–31.
Shibamoto Y, Jeremic B. Biologic premises of combined radiation therapy and chemotherapy in lung cancer. Hematol Oncol Clin North Am. 2004;18:29–40.
Bentzen SM, Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol. 2007;25:4096–103.
Murai T, Shibamoto Y, Manabe Y, Murata R, Sugie C, Hayashi A, et al. Intensity-modulated radiation therapy using static ports of tomotherapy (TomoDirect): comparison with the TomoHelical mode. Radiat Oncol. 2013;8:68.
Yang Y, Zhu Y, Cao JZ, Zhang YJ, Xu LM, Yuan ZY, et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood. 2015;126:1424–32.
Huang MJ, Jiang Y, Liu WP, Li ZP, Li M, Zhou L, et al. Early or up-front radiotherapy improved survival of localized extranodal NK/T-cell lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat Oncol Biol Phys. 2008;70:166–74.
Shibamoto Y, Yukawa Y, Tsutsui K, Takahashi M, Abe M. Variation in the hypoxic fraction among mouse tumors of different types, sizes, and sites. Jpn J Cancer Res (Gann). 1986;77:908–15.
Murata R, Shibamoto Y, Sasai K, Oya N, Shibata T, Takagi T, et al. Reoxygenation after single irradiation in rodent tumors of different types and sizes. Int J Radiat Oncol Biol Phys. 1996;34:859–65.
Wang H, Li YX, Wang WH, Jin J, Dai JR, Wang SL, et al. Mild toxicity and favorable prognosis of high-dose and extended involved-field intensity-modulated radiotherapy for patients with early-stage nasal NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2012;82:1115–21.
Shen Q, Ma X, Hu W, Chen L, Huang J, Guo Y. Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for stage I-II natural killer/T-cell lymphoma nasal type: dosimetric and clinical results. Radiat Oncol. 2013;8:152.
Manabe Y, Shibamoto Y, Sugie C, Hayashi A, Murai T, Yanagi T, et al. Helical and static-port tomotherapy using the newly-developed dynamic jaws technology for lung cancer. Technol Cancer Res Treat. 2014;14:583–91.
Kumar G, Rawat S, Puri A, Sharma MK, Chadha P, Babu AG, et al. Analysis of dose-volume parameters predicting radiation pneumonitis in patients with esophageal cancer treated with 3D-conformal radiation therapy or IMRT. Jpn J Radiol. 2012;30:18–24.
Ken S, Vieillevigne L, Franceries X, Simon L, Supper C, Lotterie JA, et al. Integration method of 3D MR spectroscopy into treatment planning system for glioblastoma IMRT dose painting with integrated simultaneous boost. Radiat Oncol. 2013;8:1.
Acknowledgments
We thank all of the patients who participated in the present study and Dr. Natsuko Takama, Dr. Toru Matsui, Dr. Yoshihiko Manabe and Dr. Takeshi Tamura for helping this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
About this article
Cite this article
Hattori, Y., Murai, T., Iwata, H. et al. Chemoradiotherapy for localized extranodal natural killer/T-cell lymphoma, nasal type, using a shrinking-field radiation strategy: multi-institutional experience. Jpn J Radiol 34, 292–299 (2016). https://doi.org/10.1007/s11604-016-0524-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-016-0524-8