Abstract
Purpose
We evaluated diffusional changes in normal-appearing white matter (NAWM) regions remote from multiple sclerosis (MS) plaques by using diffusional kurtosis imaging (DKI) to investigate the non-Gaussian behavior of water diffusion.
Materials and methods
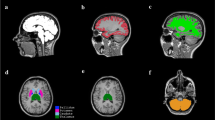
Participants were 11 MS patients and 6 age-matched healthy volunteers. DKI was performed on a 3-T MR imager. Fractional anisotropy (FA), apparent diffusion coefficient (ADC), and diffusional kurtosis (DK) maps were computed. Regions of interest (ROIs) were compared in 24 cerebral regions, including the frontal, parietal, and temporal lobe white matter (WM) in controls and NAWM in MS patients.
Results
The mean FA of all ROIs was 0.468 ± 0.014 (SD) (controls) or 0.431 ± 0.029 (MS group) (P = 0.016). Mean ADC was 0.785 ± 0.034 × 10−3 mm2/s (controls) or 0.805 ± 0.041 × 10−3 mm2/s (MS group). The mean DK of all ROIs was 0.878 ± 0.020 (controls) or 0.823 ± 0.032 (MS group) (P = 0.002). Analysis of individual ROIs revealed significant differences in DK in 3 ROIs between normal WM and NAWM, but significant differences in ADC and FA in only one ROI each.
Conclusion
DKI may be a new sensitive indicator for detecting tissue damage in MS patients in addition to conventional diffusional evaluations, for example diffusion tensor imaging.


Similar content being viewed by others
References
Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–46.
Rocca MA, Cercignani M, Iannucci G, Comi G, Filippi M. Weekly diffusion-weighted imaging of normal-appearing white matter in MS. Neurology. 2000;55:882–4.
Cercignani M, Iannucci G, Filippi M. Diffusion-weighted imaging in multiple sclerosis. Ital J Neurol Sci. 1999;20:S246–9.
Horsfield MA, Lai M, Webb SL, Barker GJ, Tofts PS, Turner R, et al. Apparent diffusion coefficients in benign and secondary progressive multiple sclerosis by nuclear magnetic resonance. Magn Reson Med. 1996;36:393–400.
Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–44.
Guo AC, MacFall JR, Provenzale JM. Multiple sclerosis: diffusion tensor MR imaging for evaluation of normal-appearing white matter. Radiology. 2002;222:729–36.
Filippi M, Agosta F. Imaging biomarkers in multiple sclerosis. J Magn Reson Imaging. 2010;31:770–88.
Rovaris M, Agosta F, Pagani E, Filippi M. Diffusion tensor MR imaging. Neuroimaging Clin N Am. 2009;19:37–43.
Werring DJ, Brassat D, Droogan AG, Clark CA, Symms MR, Barker GJ, et al. The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis: a serial diffusion MRI study. Brain. 2000;123(Pt 8):1667–76.
Castriota Scanderbeg A, Tomaiuolo F, Sabatini U, Nocentini U, Grasso MG, Caltagirone C. Demyelinating plaques in relapsing-remitting and secondary-progressive multiple sclerosis: assessment with diffusion MR imaging. AJNR Am J Neuroradiol. 2000;21:862–8.
Tsuchiya K, Hachiya J, Maehara T. Diffusion-weighted MR imaging in multiple sclerosis: comparison with contrast-enhanced study. Eur J Radiol. 1999;31:165–9.
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432–40.
Lu H, Jensen JH, Ramani A, Helpern JA. Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed. 2006;19:236–47.
Hori M, Fukunaga I, Masutani Y, Taoka T, Kamagata K, Suzuki Y, et al. Visualizing non-Gaussian diffusion: clinical application of q-space imaging and diffusional kurtosis imaging of the brain and spine. Magn Reson Med Sci. 2012;11(4):221–8.
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–6.
Kurtzke JF. A new scale for evaluating disability in multiple sclerosis. Neurology. 1955;5:580–3.
Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23:698–710.
Stejeskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1965;42:288–92.
McFarlin DE, McFarland HF. Multiple sclerosis (first of two parts). N Engl J Med. 1982;307:1183–8.
McFarlin DE, McFarland HF. Multiple sclerosis (second of two parts). N Engl J Med. 1982;307:1246–51.
Rodriguez M, Siva A, Ward J, Stolp-Smith K, O’Brien P, Kurland L. Impairment, disability, and handicap in multiple sclerosis: a population-based study in Olmsted County Minnesota. Neurology. 1994;44:28–33.
Vigeveno RM, Wiebenga OT, Wattjes MP, Geurts JJ, Barkhof F. Shifting imaging targets in multiple sclerosis: from inflammation to neurodegeneration. J Magn Reson Imaging. 2012;36:1–19.
Geurts JJ, Stys PK, Minagar A, Amor S, Zivadinov R. Gray matter pathology in (chronic) MS: modern views on an early observation. J Neurol Sci. 2009;282:12–20.
Fazekas F, Barkhof F, Filippi M, Grossman RI, Li DK, McDonald WI, et al. The contribution of magnetic resonance imaging to the diagnosis of multiple sclerosis. Neurology. 1999;53:448–56.
Barkhof F, van Walderveen M. Characterization of tissue damage in multiple sclerosis by nuclear magnetic resonance. Philos Trans R Soc Lond B Biol Sci. 1999;354:1675–86.
Miki Y, Grossman RI, Udupa JK, van Buchem MA, Wei L, Phillips MD, et al. Differences between relapsing-remitting and chronic progressive multiple sclerosis as determined with quantitative MR imaging. Radiology. 1999;210:769–74.
Miki Y, Grossman RI, Udupa JK, Wei L, Polansky M, Mannon LJ, et al. Relapsing-remitting multiple sclerosis: longitudinal analysis of MR images—lack of correlation between changes in T2 lesion volume and clinical findings. Radiology. 1999;213:395–9.
Phillips MD, Grossman RI, Miki Y, Wei L, Kolson DL, van Buchem MA, et al. Comparison of T2 lesion volume and magnetization transfer ratio histogram analysis and of atrophy and measures of lesion burden in patients with multiple sclerosis. AJNR Am J Neuroradiol. 1998;19:1055–60.
Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626–32.
Bammer R, Augustin M, Strasser-Fuchs S, Seifert T, Kapeller P, Stollberger R, et al. Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Magn Reson Med. 2000;44:583–91.
Abdallah CG, Tang CY, Mathew SJ, Martinez J, Hof PR, Perera TD, et al. Diffusion tensor imaging in studying white matter complexity: a gap junction hypothesis. Neurosci Lett. 2010;475:161–4.
Lazar M, Jensen JH, Xuan L, Helpern JA. Estimation of the orientation distribution function from diffusional kurtosis imaging. Magn Reson Med. 2008;60:774–81.
Iraji A, Davoodi-Bojd E, Soltanian-Zadeh H, Hossein-Zadeh GA, Jiang Q. Diffusion kurtosis imaging discriminates patients with white matter lesions from healthy subjects. In: Proceedings of the 33rd international conference of the IEEE engineering in medicine and biology society. 2011. p. 2796–9.
Falangola MF, Jensen JH, Babb JS, Hu C, Castellanos FX, Di Martino A, et al. Age-related non-Gaussian diffusion patterns in the prefrontal brain. J Magn Reson Imaging. 2008;28:1345–50.
Cheung MM, Hui ES, Chan KC, Helpern JA, Qi L, Wu EX. Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. Neuroimage. 2009;45:386–92.
Raab P, Hattingen E, Franz K, Zanella FE, Lanfermann H. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology. 2010;254:876–81.
Grossman EJ, Ge Y, Jensen JH, Babb JS, Miles L, Reaume J, et al. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J Neurotrauma. 2012;29:2318–27.
Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage. 2012;59:467–77.
Helpern JA, Adisetiyo V, Falangola MF, Hu C, Di Martino A, Williams K, et al. Preliminary evidence of altered gray and white matter microstructural development in the frontal lobe of adolescents with attention-deficit hyperactivity disorder: a diffusional kurtosis imaging study. J Magn Reson Imaging. 2011;33:17–23.
Hori M, Aoki S, Fukunaga I, Suzuki Y, Masutani Y. A new diffusion metric, diffusion kurtosis imaging, used in the serial examination of a patient with stroke. Acta Radiol Sh Rep. 2012;1:2.
Trampel R, Jensen JH, Lee RF, Kamenetskiy I, McGuinness G, Johnson G. Diffusional kurtosis imaging in the lung using hyperpolarized 3He. Magn Reson Med. 2006;56:733–7.
Allen IV, McQuaid S, Mirakhur M, Nevin G. Pathological abnormalities in the normal-appearing white matter in multiple sclerosis. Neurol Sci. 2001;22:141–4.
Acknowledgments
This study was partly supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yoshida, M., Hori, M., Yokoyama, K. et al. Diffusional kurtosis imaging of normal-appearing white matter in multiple sclerosis: preliminary clinical experience. Jpn J Radiol 31, 50–55 (2013). https://doi.org/10.1007/s11604-012-0147-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-012-0147-7