Abstract
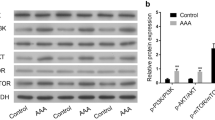
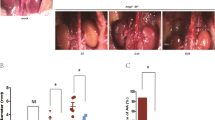
The aim of the present study is to address the effect of rapamycin on abdominal aortic aneurysm (AAA) and the potential mechanisms. A clinically relevant AAA model was induced in apolipoprotein E-deficient (ApoE-/-) mice, in which miniosmotic pump was implanted subcutaneously to deliver angiotensin II (Ang II) for 14 days. Male ApoE-/- mice were randomly divided into 3 groups: saline infusion, Ang II infusion, and Ang II infusion plus intraperitoneal injection of rapamycin. The diameter of the supra-renal abdominal aorta was measured by ultrasonography at the end of the infusion. Then aortic tissue was excised and examined by Western blotting and histoimmunochemistry. Ang n with or without rapamycin treatment was applied to the cultured vascular smooth muscle cells (VSMCs) in vitro. The results revealed that rapamycin treatment significantly attenuated the incidence of Ang II induced-AAA in ApoE-/- mice. Histologic analysis showed that rapamycin treatment decreased disarray of elastin fibers and VSMCs hyperplasia in the medial layer. Immunochemistry staining and Western blotting documented the increased phospho-ERK1/2 and ERK1/2 expression in aortic walls in Ang II induced-AAA, as well as in human lesions. Whereas in the rapamycintreated group, decreased phospho-ERKl/2 expression level was detected. Moreover, rapamycin reversed Ang II -induced VSMCs phenotypic change both in vivo and in vitro. Based on those results, we confirmed that rapamycin therapy suppressed Ang II -induced AAA formation in mice, partially via VSMCs phenotypic modulation and down-regulation of ERK1/2 activity.
Similar content being viewed by others
References
Golledge J, Norman PE. Current status of medical management for abdominal aortic aneurysm. Atherosclerosis, 2011,217(1): 57–63
Nordon IM, HinchlifFe RJ, Loftus IM, etal. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol, 2011, 8(2): 92–102
Wang Y, Ait-Oufella H, Herbin O, etal. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin Il-infused mice. J Clin Invest, 2010, 120(2): 422–432
Ghoshal S, Loftin CD. Cyclooxygenase-2 inhibition attenuates abdominal aortic aneurysm progression in hyperlipidemic mice. PLoS One, 2012,7(1l): e44 369
Cafueri G, Parodi F, Pistorio A, etal. Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PLoS One, 2012,7(4): e35 312
Satoh K, Nigra P, Matoba T, etal. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med, 2009, 15(6): 649–656
Johanning JM, Franklin DP, Han DC, etal. Inhibition of inducible nitric oxide synthase limits nitric oxide production and experimental aneurysm expansion. J Vase Surg, 2001, 33(3): 579–586
Ailawadi G, Eliason JL, Upchurch GR, Jr. Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vase Surg, 2003, 38(3): 584–588
Patel MI, Ghosh P, Melrose J, etal. Smooth muscle cell migration and proliferation is enhanced in abdominal aortic aneurysms. Aust N Z J Surg, 1996, 66(5): 305–308
Mao N, Gu T, Shi E, etal. Phenotypic switching of vascular smooth muscle cells in animal model of rat thoracic aortic aneurysm. Interact Cardiovasc Thorac Surg, 2015,21(1): 62–70
Ailawadi G, Moehle CW, Pei H, etal. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg, 2009, 138(6): 1392–1399
Savoia C, Burger D, Nishigaki N, etal. Angiotensin II and the vascular phenotype in hypertension. Expert Rev Mol Med, 2011,13:ell
Eguchi S, Dempsey PJ, Frank GD, etal. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J Biol Chem, 2001, 276(11): 7957–7962
Daugherty A, Cassis L. Angiotensin II and abdominal aortic aneurysms. Curr Hypertens Rep, 2004, 6(6): 442–446
Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest, 2000, 105(11): 1605–1612
Thompson RW. Reflections on the pathogenesis of abdominal aortic aneurysms. Cardiovasc Surg, 2002, 10(4): 389–394
McCormick ML, Gavrila D, Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vase Biol, 2007, 27(3): 461–469
Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev, 2004, 18(16): 1926–1945
Lipton JO, Sahin M. The neurology of mTOR. Neuron, 2014, 84(2): 275–291
Holmes DR Jr, Leon MB, Moses JW, etal. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation, 2004, 109(5): 634–640
Khan W, Farah S, Domb AJ. Drug eluting stents: developments and current status. J Control Release, 2012, 161(2): 703–712
Li W, Li Q, Qin L, etal. Rapamycin inhibits smooth muscle cell proliferation and obstructive arteriopathy attributable to elastin deficiency. Arterioscler Thromb Vase Biol, 2013, 33(5): 1028–1035
Kim JA, Jang HJ, Martinez-Lemus LA, etal. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab, 2012,302(2): E201-208
Hafizi S, Wang X, Chester AH, etal. ANG II activates effectors of mTOR via PI3-K signaling in human coronary smooth muscle cells. Am J Physiol Heart Circ Physiol, 2004,287(3): H1232-1238
Martin KA, Rzucidlo EM, Merenick BL, etal. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol, 2004,286(3): C507-517
Zuckermann A, Keogh A, Crespo-Leiro MG, etal. Randomized controlled trial of sirolimus conversion in cardiac transplant recipients with renal insufficiency. Am J Transplant, 2012, 12(9): 2487–2497
Ray JL, Leach R, Herbert JM, etal. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sei, 2001, 23(4): 185–188
Rodriguez-Vita J, Sanchez-Lopez E, Esteban V, etal. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation, 2005,1ll(19): 2509–2517
Ghosh A, DiMusto PD, Ehrlichman LK, etal. The role of extracellular signal-related kinase during abdominal aortic aneurysm formation. J Am Coll Surg, 2012, 215(5): 668–680.el
Moran CS, Jose RJ, Moxon JV, etal. Everolimus limits aortic aneurysm in the apolipoprotein E-deficient mouse by downregulating C-C chemokine receptor 2 positive monocytes. Arterioscler Thromb Vase Biol, 2013,33(4): 814–821
Lawrence DM, Singh RS, Franklin DP, etal. Rapamycin suppresses experimental aortic aneurysm growth. J Vase Surg, 2004, 40(2): 334–338
Lesauskaite V, Tanganelli P, Sassi C, etal. Smooth muscle cells of the media in the dilatative pathology of ascending thoracic aorta: morphology, immunoreactivity for osteopontin, matrix metalloproteinases, and their inhibitors. Hum Pathol, 2001, 32(9): 1003–1011
Martin KA, Merenick BL, Ding M, etal. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem, 2007, 282(49): 36 112–36 120
Hayashi K, Takahashi M, Kimura K, etal. Changes in the balance of phosphoinositide 3-kinase/protein kinase B (Akt) and the mitogen-activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells. J Cell Biol, 1999,145(4):727–740
Kyaw M, Yoshizumi M, Tsuchiya K, etal. Antioxidants inhibit JNK and p38MAPK activation but not ERK1/2 activation by angiotensin IT in rat aortic smooth muscle cells. Hypertens Res, 2001, 24(3): 251–261
Griendling KK, Minieri CA, Ollerenshaw JD, etal. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res, 1994, 74(6): 1141–1148
Sano M, Fukuda K, Sato T, etal. ERK and p38 МАРК, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res, 2001, 89(8): 661–669
Wilkie N, Morton C, Ng LL, etal. Stimulated mitogen-activated protein kinase is necessary but not sufficient for the mitogenic response to angiotensin II. A role for phospholipase D. J Biol Chem, 1996, 271(50): 32 447–32 453
Holm TM, Habashi JP, Doyle JJ, etal. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science, 2011, 332(6027): 358–361
Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci, 2011, 36(6): 320–328
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by grants from the National Natural Science Foundation of China (No. 81570325), and the Fundamental Research Funds for the Central Universities.
Rights and permissions
About this article
Cite this article
Li, Ff., Shang, Xk., Du, Xl. et al. Rapamycin Treatment Attenuates Angiotensin II -induced Abdominal Aortic Aneurysm Formation via VSMC Phenotypic Modulation and Down-regulation of ERK1/2 Activity. CURR MED SCI 38, 93–100 (2018). https://doi.org/10.1007/s11596-018-1851-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-018-1851-z