Summary
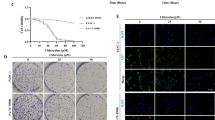
Neamine, a non-toxic derivative of neomycin, has recently been shown to have antitumor activities in various types of cancers. However, its effect on pancreatic cancer is still unknown. The study aimed to investigate its antitumor activity on pancreatic cancer and the underlying mechanisms. MTT assay was used to observe the effect of neamine on angiogenin (ANG)-induced AsPC-1 cell proliferation. Tissue microassay and immunofluorescence staining were used to detect the expression of ANG and its nuclear translocation, respectively. Tumor xenografts were established by subcutaneous inoculation of AsPC-1 pancreatic cancer cells into the right flanks of nude mice, and neamine was injected subcutaneously. Immunohistochemistry was done to observe the expression of ANG, CD31 and Ki-67 in tumor xenografts. It was found that neamine blocked the nuclear translocation of ANG effectively and inhibited ANG-induced AsPC-1 cell proliferation in a dose-dependent manner. Neamine had anti-tumor effects on AsPC-1 xenograft models. Consistently, neamine reduced the expression levels of ANG, Ki-67 and CD31 in tumor xenografts. It was concluded that neamine may be a promising agent for treatment of pancreatic cancer.
Similar content being viewed by others
References
Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet, 2011,378(9791):607–620
Siegel R, Ma J, Zou Z, et al. Cancer statistics. CA cancer J Clin, 2014,64(1):9–29
Liu SX, Xia ZS, Zhong YQ. Genetic therapy in pancreatic cancer. World J Gastroenterol, 2014,20(37): 13343–13368
Burris H, Storniolo A. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer, 1997,33(suppl): S18–S22
Seo Y, Baba H, Fukuda T, et al. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer, 2000,88(10): 2239–2245
Fujimoto K, Hosotani R, Wada M, et al. Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer, 1998,34(9):1439–1447
Wang H, Chen Q. Expression and significance of cyclooxygenase-2 in human pancreatic carcinomas. Chin Ger J Clin Oncol, 2005,22(2):121–123
Fett JW, Strydom DJ, Lobb RR, et al. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry, 1985,24(20): 5480–5486
Li R, Riordan JF, Hu G. Nuclear translocation of human angiogenin in cultured human umbilical artery endothelial cells is microtubule and lysosome independent. Biochem Biophys Res Commun, 1997,238 (238):305–312
Moroianu J, Riordan JF. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc Natl Acad Sci USA, 1994,91(5):1677–1681
Kishimoto K, Liu S, Tsuji T, et al. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene, 2005, 24(3):445–456
Bottero V, Sadagopan S, Johnson KE, et al. Kaposi's sarcoma-associated herpesvirus-positive primary effusion lymphoma tumor formation in NOD/SCID mice is inhibited by neomycin and neamine blocking angiogenin's nuclear translocation. J Virol, 2013,87(21):11806–11820
Shimoyama S, Gansauge F, Gansauge S, et al. Increased angiogenin expression in pancreatic cancer is related to cancer aggressiveness. Cancer Res, 1996,56(12):2703–2706
Olson KA, Fett JW, French TC, et al. Angiogenin antagonists prevent tumor growth in vivo. Proc Natl Acad Sci USA, 1995,92(2):442–446
Olson KA, French TC, Vallee BL, et al. A monoclonal antibody to human angiogenin suppresses tumor growth in athymic mice. Cancer Res, 1994,54(17): 4576–4579
Olson KA, Byers HR, Key ME, et al. Prevention of human prostate tumor metastasis in athymic mice by antisense targeting of human angiogenin. Clin Cancer Res, 2001,7(11):3598–3605
Sadagopan S, Sharma-Walia N, Veettil MV, et al. Kaposi's sarcoma-associated herpesvirus upregulates angiogenin during infection of human dermal microvascular endothelial cells, which induces 45S rRNA synthesis, antiapoptosis, cell proliferation, migration, and angiogenesis. J Virol, 2009,83(7): 3342–3364
Hu GF. Neomycin inhibits angiogenin-induced angiogenesis. Proc Natl Acad Sci USA, 1998,95:9791–9795
Williams P, Bennett D, Gleason C, et al. Correlation between renal membrane binding and nephrotoxicity of aminoglycosides. Antimicrob Agents Chemother, 1987,31(4):570–574
Zhao J, Wang YC, Yang LY, et al. Neamine inhibits cell proliferation, migration, and invasion in H7402 human hepatoma cells. Saudi Med J, 2010,31(12):1309–1314
Kishimoto K, Yoshida S, Ibaragi S, et al. Neamine inhibits oral cancer progression by suppressing angiogenin-mediated angiogenesis and cancer cell proliferation. Anticancer Res, 2014,34(5):2113–2121
Yuan Y, Wang F, Liu XH, et al. Angiogenin is involved in lung adenocarcinoma cell proliferation and angiogenesis. Lung Cancer, 2009,66(1):28–36
Hirukawa S, Olson KA, Tsuji T, et al. Neamine inhibits xenografic human tumor growth and angiogenesis in athymic mice. Clin Cancer Res, 2005, 11(24 Pt 1): 8745–8752
Ibaragi S, Yoshioka N, Li S, et al. Neamine inhibits prostate cancer growth by suppressing angiogeninmediated rRNA transcription. Clin Cancer Res, 2009, 15(6):1981–1988
Yaping Liu, Xiaoyan Zhang, Songlin An, et al. Pharmacokinetics of neamine in rats and anti-cervical cancer activity in vitro and in vivo. Cancer Chemother Pharmacol, 2015, 75(3):465–474
Liu YP, Hu GF, Wu YX. Neamine is preferential as an anti-prostate cancer reagent by inhibiting cell proliferation and angiogenesis with lower toxicity than cis-platinum. Oncol Lett, 2015,10(1):137–142
Deng SR, Li J, Zhang ZQ, et al. DS147 improves pregnancy in mice with embryo implantation dysfunction induced by controlled ovarian stimulation. J Huazhong Univ Sci Technolog Med Sci, 2013,33(4):573–580
Saif MW. Anti-angiogenesis therapy in pancreatic carcinoma. Jop, 2006,7(2):163–173
Tsuji T, Sun Y, Kishimoto K, et al. Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer Res, 2005,65(4):1352–1360
Sathish S, Mohanan VV, Nitika P, et al. Kaposi's sarcoma-associated herpesvirus-induced angiogenin plays roles in latency via the phospholipase C gamma pathway: blocking angiogenin inhibits latent gene expression and induces the lytic cycle. J Virol, 2011,85(6):2666–2685
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the National Major Special Project of the Ministry of Science and Technology of China (No. 2012ZX09103101047), the National Natural Science Foundation of China (No. 81373873) and the Special Fund for Basic Scientific Research of Central College of China (No. 2014QN129).
Rights and permissions
About this article
Cite this article
Liu, Yp., Wu, Yl., Zhang, Xy. et al. Neamine inhibits growth of pancreatic cancer cells In Vitro and In Vivo . J. Huazhong Univ. Sci. Technol. [Med. Sci.] 36, 82–87 (2016). https://doi.org/10.1007/s11596-016-1546-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-016-1546-2