Summary
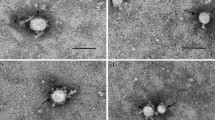
Microvesicles (MVs) are the heterogeneous mixtures of vesicles. MVs released by leukemia cells constitute an important part of the leukemia microenvironment. MVs might act as important reservoirs of microRNAs (miRNAs). It is worth evaluating whether MVs possess some unique miRNA contents that are valuable in understanding the pathogenesis. In this study, we investigated the miRNA expression patterns of Nalm-6-derived MVs, Jurkat-derived MVs and normal cell-derived MVs using miRNA microarrays. The potential target genes regulated by differentially expressed miRNAs were also predicted and analyzed. Results demonstrated that 182 miRNAs and 166 miRNAs were differentially expressed in Nalm-6-MVs and Jurkat-MVs, respectively. Many oncogenes, tumor suppressors and signal pathway genes were targeted by these aberrantly expressed miRNAs, which might contribute to the development of B-ALL or T-ALL. Our findings expanded the potential diagnostic markers of ALL and provided useful information for ALL pathogenesis.
Similar content being viewed by others
References
Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood, 1997,89(4):1121–1132
Holme PA, Orvim U, Hamers MJ, et al. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler Thromb Vasc Biol, 1997,17(4):646–653
Aupeix K, Hugel B, Martin T, et al. The significance of shed membrane particles during programmed cell death in vitro, and in vivo, in HIV-1 infection. J Clin Invest, 1997,99(7):1546–1554
Ratajczak J, Wysoczynski M, Hayek F, et al. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia, 2006,20(9):1487–1495
Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, et al. Tumour-derived microvesicles modulate biological activity of human monocytes. Immunol Lett, 2007,113(2):76–82
Bartel DP. MiRNAs: genomics, biogenesis, mechanism, and function. Cell, 2004,116(2):281–297
Lu J, Getz G, Miska EA, et al. MiRNA expression profiles classify human cancers. Nature, 2005,354(7043):834–838
Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and MiRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol, 2007,9(6): 654–659
Rowley JD. Molecular genetics in acute leukemia. Leukemia, 2000,14(3):513–517
Thiel E, Kranz BR, Raghavachar A. Prethymic phenotype and genotype of pre-T(CD7+/ER−)-cell leukemia and its clinical significance within adult acute lymphoblastic leukemia. Blood, 1989,73(5):1247–1258
Zhou B, Wang S, Mayr C, et al. miR-150, a miRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci U S A, 2007,104(17):7080–7085
Martínez MC, Larbret F, Zobairi F, et al. Transfer of differentiation signal by membrane microvesicles harboring hedgehog morphogens. Blood, 2006,108(9):3012–3020
Zhang ZK, Davies KP, Allen J. Cell cycle arrest and repression of cyclin D1 transcription by INI1/hSNF5. Mol Cell Biol, 2002,22(16):5975–5988
Le Baccon P, Leroux D, Dascalescu C, et al. Novel evidence of a role for chromosome 1 pericentric heterochromatin in the pathogenesis of B-cell lymphoma and multiple myeloma. Genes Chromosomes Cancer, 2001,32(3):250–264
Nagel S, Venturini L, Przybylski GK, et al. Activation of miR-17-92 by NK-like homeodomain proteins suppresses apoptosis via reduction of E2F1 in T-cell acute lymphoblastic leukemia. Leuk Lymphoma, 2009,50(1):101–108
Molitoris JK, McColl KS, Distelhorst CW. Glucocorticoid-mediated repression of the oncogenic MiRNA cluster miR-17∼92 contributes to the induction of Bim and initiation of apoptosis. Mol Endocrinol, 2011,25(3):409–420
Grillari J, Hackl M, Grillari-Voglauer R. miR-17-92 cluster: ups and downs in cancer and aging. Biogerontology, 2010,11(4):501–506
Martinez-Delgado B, Meléndez B, Cuadros M, et al. Expression profiling of T-cell lymphomas differentiates peripheral and lymphoblastic lymphomas and defines survival related genes. Clin Cancer Res, 2004,10(15):4971–4982
Bouillet P, Purton JF, Godfrey DI. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature, 2002,415(6874):922–926
Glittenberg M, Ligoxygakis P. CYLD: a multifunctional deubiquitinase. Fly (Austin), 2007,1(6):330–332
Kraszewska MD, Dawidowska M, Kosmalska M. BCL11B, FLT3, NOTCH1 and FBXW7 mutation status in T-cell acute lymphoblastic leukemia patients. Blood Cells Mol Dis, 2013,50(1):33–38
Giambra V, Jenkins CR, Wang H. NOTCH1 promotes T cell leukemia-initiating activity by RUNX-mediated regulation of PKC-θ and reactive oxygen species. Nat Med, 2012, 18(11):1693–1698.
Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis, 2006,36(2):315–321
Sassen S, Miska EA, Caldas C. MiRNA: implications for cancer. Virchows Arch, 2008,452(1):1–10
Zhu YD, Wang L, Sun C, et al. Distinctive MiRNA signature is associated with the diagnosis and prognosis of acute leukemia. Med Oncol, 2012,29(4):2323–2331
Zhang Y, Liao JM, Zeng SX, et al. p53 downregulates Down syndrome-associated DYRK1A through miR-1246. EMBO Rep, 2011,12(8):811–817
Piepoli A, Tavano F, Copetti M, et al. miRNA expression profiles identify drivers in colorectal and pancreatic cancers. PLoS One, 2012,7(3):e33663
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (No. 81170462).
Rights and permissions
About this article
Cite this article
Li, Wy., Chen, Xm., Xiong, W. et al. Detection of microvesicle miRNA expression in ALL subtypes and analysis of their functional roles. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 34, 640–645 (2014). https://doi.org/10.1007/s11596-014-1330-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-014-1330-0