Summary
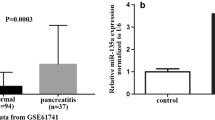
This study examined the expressions of miR-22 and miR-135a in rats with acute edematous pancreatitis (AEP) and their target genes in order to shed light on the involvement of miR-22 and miR-135a in the pathogenesis of acute pancreatitis (AP). The in vivo model of AEP was established by introperitoneal injection of L-arginine (150 mg/kg) in rats. The miRNA microarray analysis was used to detect the differential expression of miRNAs in pancreatic tissue in AEP and normal rats. The in vitro AEP model was established by inducing the rat pancreatic acinar cell line (AR42J) with 50 ng/mL recombinant rat TNF-α. Real-time quantitative RT-PCR was employed to detect the expression of miR-22 and miR-135a in AR42J cells. Lentiviruses carrying the miRNA mimic and anti-miRNA oligonucleotide (AMO) of miR-22 and miR-135a were transfected into the AR42J cells. The AR42J cells transfected with vehicle served as control. Western blotting was used to measure the expression of activated caspase3 and flow cytometry analysis to detect the apoptosis of AR42J cells. Targets of miR-22 and miR-135a were predicted by using TargetScan, miRanda, and TarBase. Luciferase reporter assay and quantitative real-time RT-PCR were performed to confirm whether ErbB3 and Ptk2 were the target gene of miR-22 and miR-135a, respectively. The results showed that the expression levels of miR-22 and miR-135a were obviously increased in AEP group compared with the control group in in-vivo and in-vitro models. The expression levels of miR-22 and miR-135a were elevated conspicuously and the expression levels of their target genes were reduced significantly in AR42J cells transfected with lentiviruses carrying the miRNA mimic. The apoptosis rate was much higher in the TNF-α-induced cells than in non-treated cells. The AR42J cells transfected with miRNA AMOs expressed lower level of miR-22 and miR-135a and had lower apoptosis rate, but the expression levels of ErbB3 and Ptk2 were increased obviously. It was concluded that the expression levels of miR-22 and miR-135a were elevated in AEP. Up-regulating the expression of miR-22 and miR-135a may promote the apoptosis of pancreatic acinar cells by repressing ErbB3 and Ptk2 expression in AEP.
Similar content being viewed by others
References
Bhatia M, Hegde A. Treatment with antileukinate, a CXCR2 chemokine receptor antagonist, protects mice against acute pancreatitis and associated lung injury. Regul Pept, 2007,138(1):40–48
Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet, 2008,371(9607):143–152
Booth DM, Mukherjee R, Sutton R, et al. Calcium and reactive oxygen species in acute pancreatitis: friend or foe? Antioxid Redox Signal, 2011,15(10):2683–2698
Bhatia M. Apoptosis of pancreatic acinar cells in acute pancreatitis: is it good or bad? J Cell Mol Med, 2004,;8(3):402–409
Kaiser AM, Saluja AK, Sengupta A, et al. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol, 1995,269(5 Pt 1):C1295–C1304
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 2004,116(2):281–297
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell, 2009,136(2):215–233
Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov, 2010,9(10):775–789
Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell, 136(4):642–655
Glisić T, Sijacki A, Vuković V, et al. Bernard Organ Failure Score in estimation of most severe forms of acute pancreatitis. Srp Arh Celok Lek, 2009,137(3–4):166–170
Mareninova OA, Sung KF, Hong P, et al. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem, 2006,281(6):3370–3381
Sandoval D, Gukovskaya A, Reavey P, et al. The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology, 1996,111(4):1081–1091
Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology, 2004, 4(6):567–586
Yilmaz M, Topsakal S, Herek O, et al. Effects of etanercept on sodium taurocholate-induced acute pancreatitis in rats. Transl Res, 2009,154(5):241–249
Sato H, Siow RC, Bartlett S, et al. Expression of stress proteins heme oxygenase-1 and-2 in acute pancreatitis and pancreatic islet betaTC3 and acinar AR42J cells. FEBS Lett, 1997,405(2):219–223
Twait E, Williard DE, Samuel I. Dominant negative p38 mitogen-activated protein kinase expression inhibits NF-kappaB activation in AR42J cells. Pancreatology, 2010,10(2–3):119–128
Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol, 1988,141(8):2629–2634
Satoh A, Gukovskaya AS, Nieto JM, et al. PKC-delta and -epsilon regulate NF-kappaB activation induced by cholecystokinin and TNF-alpha in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol, 2004,287(3):G582–G591
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol, 2001,2(2):127–137
Guy PM, Platko JV, Cantley LC, et al. Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci USA. 1994,91(17):8132–8136
Sierke SL, Cheng K, Kim HH, et al. Biochemical characterization of the protein tyrosine kinase homology domain of the ErbB3 (HER3) receptorprotein. Biochem J, 1997,322( Pt 3):757–763.
Soltoff SP, Carraway KL 3rd, Prigent SA, et al. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol, 1994,14(6):3550–3558
Kim HH, Sierke SL, Koland JG. Epidemal growth factor-dependent association of phosphatidylinositol 3-kinase withthe erbB3 gene product. J Biol Chem, 1994,269(40):24747–24755
Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov, 2005,4(12):988–1004
Chen C, Chang YC, Lan MS, et al. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol, 2013,42(3):1113–1119
Mazumdar M, Adhikary A, Chakraborty S, et al. Targeting RET to induce medullary thyroid cancer cell apoptosis: an antagonistic interplay between PI3K/Akt and p38MAPK/caspase-8 pathways. Apoptosis, 2013,18(5):589–604
Yuan Q, Cai S, Zhang X, et al. A new protoapigenone analog RY10-4 induces apoptosis and suppresses invasion through the PI3K/Akt pathway in human breast cancer. Cancer Lett, 2012,324(2):210–220
Yuan L, Wang J, Xiao H, et al. Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicol Appl Pharmacol, 2012,265(1):83–92
Kim MS, Kim JH, Bak Y, et al. 2,4-bis (p-hydroxyphenyl)-2-butenal (HPB242) induces apoptosis via modulating E7 expression and inhibition of PI3K/Akt pathway in SiHa human cervical cancer cells. Nutr Cancer, 2012,64(8):1236–1244
Harashima N, Inao T, Imamura R, et al. Roles of the PI3K/Akt pathway and autophagy in TLR3 signaling-induced apoptosis and growth arrest of human prostate cancer cells. Cancer Immunol Immunother, 2012,61(5):667–676
Zhou L, Luan H, Liu Q, et al. Activation of PI3K/Akt and ERK signaling pathways sinomenine-induced lung cancer cell apoptosis. Mol Med Rep, 2012,5(5):1256–1260
Hu W, Shen T, Wang MH. Cell cycle arrest and apoptosis induced by 3,5-dicaffepyl quinate in human colon cancer cells: Involvement of the PI3K/Aktand MAP kinase pathways. Chem Biol Interact, 2011,194(1):48–57
Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal, 2010, 5:10
Yuan Z, Zheng Q, Fan J, et al. Expression and prognostic significance of focal adhesion kinase in hepatocellular carcinoma. J Cancer Res Clin Oncol, 2010,136(10):1489–1496
Chen JS, Huang XH, Wang Q, et al. FAK is involved in invasion and metastasisof hepatocellular carcinoma. Clin Exp Metastasis, 2010,27(2):71–82
Ward KK, Tancioni I, Lawson C, et al. Inhibition of focal adhesion kinase (FAK) activity prevents anchorage-independent ovarian carcinoma cell growth and tumor progression. Clin Exp Metastasis, 2013,30(5):579–594
Ucar DA, Kurenova E, Garrett TJ, et al. Disruption of the protein interaction between FAK and IGF-1R inhibits melanoma tumor growth. Cell Cycle, 2012,11(17):3250–3259
Xie P, Kondeti VK, Lin S, et al. Role of extracellular matrix renal tubulo-interstitial nephritis antigen (TINag) in cell survival utilizing integrin (alpha)vbeta3/focal adhesion kinase (FAK)/phosphatidylinositol 3-kinase (PI3K)/protein kinase B-serine/threonine kinase (AKT) signaling pathway. J Biol Chem, 2011,286(39):34131–34146
Alisi A, Arciello M, Petrini S, et al. Focal adhesion kinase (FAK) mediates the induction of pro-oncogenic and fibrogenic phenotypes in hepatitis Cvirus (HCV)-infected cells. PLoS One, 2012,7(8):e44147
Shi H, Lee YS, Lee YC. Sodium butyrate-induced DAPK-mediated apoptosis in human gastric cancer cells. Oncol Rep, 2012,27(4):1111–1115
Shi H, Liu H, Zhao G. Effect of small interfering RNA transfection on FAK and DLC1 mRNA expression in OVCAR-3. Mol Biol Rep, 2012,39(10):9299–9306
Author information
Authors and Affiliations
Corresponding author
Additional information
Both authors contributed equally to this work.
The project was supported by grants from the National Natural Science Foundation of China (Nos.31140078, and 30972928).
Rights and permissions
About this article
Cite this article
Qin, T., Fu, Q., Pan, Yf. et al. Expressions of miR-22 and miR-135a in acute pancreatitis. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 34, 225–233 (2014). https://doi.org/10.1007/s11596-014-1263-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-014-1263-7