Summary
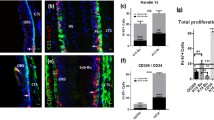
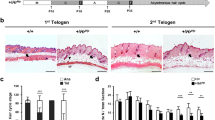
In the bulge region of the hair follicle, a densely and concentrically packed cell mass is encircled by the arrector pili muscle (APM), which offers a specilized microenvironment (niche) for housing heterogeneous adult stem cells. However, the detailed histological architecture and the cellular composition of the bulge region warrants intensive study and may have implications for the regulation of hair follicle growth regulation. This study was designed to define the gene-expression profiles of putative stem cells and lineage-specific precursors in the mid-portions of plucked hair follicles prepared according to the presence of detectable autofluorescence. The structure was also characterized by using a consecutive sectioning technique. The bulge region of the hair follicle with autofluorescence was precisely excised by employing a micro-dissection procedure. Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed to identify the gene expression profiles specific for epithelial, melanocyte and stromal stem cells in the bulge region of the hair follicle visualized by autofluorescence. The morphology and its age-dependent changes of bulge region of the hair follicles with autofluorescence segment were also examined in 9 scalp skin specimens collected from patients aged 30 weeks to 75 years, by serial sectioning and immuno-staining. Gene expression profile analysis revealed that there were cells with mRNA transcripts of DctHiTyraseLo-Tyrp1LoMC1RLoMITFLo/K15Hi/NPNTHi in the bulge region of the hair follicle with autofluorescence segments, which differed from the patterns in hair bulbs. Small cell-protrusions that sprouted from the outer root sheath (ORS) were clearly observed at the APM inserting level in serial sections of hair follicles by immunohistological staining, which were characteristically replete with K15+/K19+expressing cells. Likewise, the muscle bundles of APM positive for smooth muscle actin intimately encircled these cell-protrusions, and the occurrence frequency of the cell-protrusions was increased in fetal scalp skin compared with adult scalp skin. This study provided the evidence that the cell-protrusions occurring at the ORS relative to the APM insertion are more likely to be characteristic of the visible niches that are filled with abundant stem cells. The occurrence frequency of these cell-protrusions was significantly increased in fetal scalp skin samples (128%) as compared with the scalp skins of younger (49.4%) and older (25.4%) adults (P<0.01), but difference in the frequency between the two adult groups were not significant. These results indicated that these cell-protrusions function as a niche house for the myriad stem cells and/or precursors to meet the needs of the development of hair follicles in an embryo. The micro-dissection used in this study was simple and reliable in excising the bulge region of the hair follicle with autofluorescence segments dependent on their autofluorescence is of value for the study of stem cell culture.
Similar content being viewed by others
References
Moore KA, Lemischka IR. Stem cells and their niches. Science, 2006,311(5679):1880–1885
Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: Stem cells and their niche. Cell, 2004,116(6): 769–778
Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell, 1990,619(7):1329–1337
Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol, 2005,3(11):e331
Ohyama M, Terunuma A, Tock CL, et al. Characteriza tion and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest, 2006,116(1):249–260
Wu BP, Tao Q, Lyle S. Autofluorescence in the stem cell region of the hair follicle bulge. J Invest Dermatol, 2005, 124(4):860–862
Voog J, Jones DL. Stem cells and the niche: A dynamic duo. Cell Stem Cell, 2010, 6(2):103–115
Poblet E, Jimenez F, Godinez JM, et al. The immunohistochemical expression of CD34 in human hair follicles: a comparative study with the bulge marker CK15. Clin Exp Dermatol, 2006,31(6):807–812
Alonso, Fuchs E. Stem cells of the skin epithelium. Proc Natl Aced Sci USA, 2003,100(suppl 1):11830–11835
Meyer KC, Klatte JE, Dinh HV, et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol, 2008,159(5):1077–1085
Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med, 2005,11(12):1351–1354
Tanimura S, Tadokoro Y, Inomata K, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell, 2011,8(2):177–187
Yu H, Kumar SM, Kossenkov AV, et al. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol, 2010, 130(5):1227–1236
Tiede S, Kloepper JE, Whiting DA, et al. The ‘follicular trochanter’: an epithelial compartment of the human hair follicle bulge region in need of further characterization. Br J Dermatol, 2007,157(5):1013–1016
Zhao ZS, Li L, Wang HJ, et al. Expression and prognostic signiflcance of CEACAM6, ITGB1, and CYR61 in peripheral blood of patients with gastric cancer. J Surg Oncol, 2011,104(5):525–529
Fujiwara H, Ferreira M, Donati G, et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell, 2011,144(4):577–589
Sviderskaya EV, Easty DJ, Lawrence MA, et al. Functional neurons and melanocytes induced from immortal lines of postnatal neural crest-like stem cells. FASEB J, 2009,23(9):3179–3192
Larouche D, Tong X, Fradette J, et al. Vibrissa hair bulge houses two populations of skin epithelial stem cells distinct by their keratin profile. FASEB J, 2008, 22(5): 1404–1415
Kloepper JE, Hendrix S, Bodó E, et al. Functional role of beta 1 integrin-mediated signalling in the human hair follicle. Exp Cell Res, 2008, 314(3):498–508
Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest, 2001,107(4):409–417
Rabbani P, Takeo M, Chou W, et al. Coordiated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell, 2011, 145(6):941–955
Takeda N, Jain R, LeBoeuf MR, et al. Interconversion between intestinal stem cell populations in distinct niches. Science, 2011,334(6061):1420–1424
Nath M, Offers M, Hummel M, et al. Isolation and in vitro expansion of Lgr6-positive multipotent hair follicle stem cells. Cell Tissue Res, 2011, 344(3):435–444
Yonetani S, Moriyama M, Nishigori C, et al. In vitro expansion of immunature melanoblasts and their ability to repopulate melanocyte stem cells in the hair follicle. J Invest Dermatol, 2008,128(2):408–420
Meyer-Blazejewska EA, Call MK, Yamanaka O, et al. From hair to cornea: toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells, 2011,29(1):57–66
Zhao J, Liu LQ, Wang YJ, et al. Treatment of alopecia by transplantation of hair follicle stem cells and dermal papilla cells encapsulated in alginate gels. Med Hypotheses, 2008,70(5):1014–1016
Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell, 2011,19(2):257–272
Barcaui CB, Pineiro-Maceira J, De Avelar Alchorne MM. Arrector pili muscle: evidence of proximal attachment variant in terminal follicles of the scalp. Br J Dermatol, 2002,146(4):657–658
Song WC, Hwang WJ, Shin C, et al. A new model for the morphology of the arrector pili muscle in the follicular unit based on three-dimensional reconstruction. J Anat, 2006,208(5):643–648
Song WC, Hu KS, Koh KS. Multiunit arrector pili muscular structure as a variation observed by using computer-based three-dimensional reconstruction. Cell Tissue Res, 2005,322(2):335–337
Enshell-Seijffers D, Lindon C, Kashiwagi M, et al. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell, 2010, 18(4):633–642
Oh HS, Smart RC. An estrogen receptor pathway regulates the telogen-anagen hair follicle transition and influences epidermal cell proliferationm. Proc Natl Acad Sci USA, 1996,93(22):12 525–12 530
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by grants from the National Natural Science Foundation of China (No. 8107138) and a CMA-LOreal China Hair Grant (No. H2010040414).
Rights and permissions
About this article
Cite this article
Wang, X., Shi, Y., Zhou, Q. et al. Detailed histological structure of human hair follicle bulge region at different ages: A visible niche for nesting adult stem cells. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 32, 648–656 (2012). https://doi.org/10.1007/s11596-012-1012-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-012-1012-8