Abstract
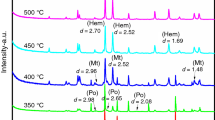
To explore the effects of mechanical activation methods (ball mill, planetary mill and rod mill) on the oxidation and the spontaneous combustion of pyrite, the kinetic curves of non-activated pyrite and mechanically activated pyrite were created by simultaneous thermal analysis. The structural characteristics and changes of mechanically activated pyrite were investigated by X-ray diffraction and SEM, and the relationship between the mean diameter and the grinding time was obtained by using a laser particle size analyzer. The kinetic model of pyrite and the kinetic parameters were deduced using Bagchi method. The relationship between the kinetic parameters indicates that, pyrite activated by ball milling shows the best thermal stability at the same diameter. By comparing and analyzing the X-ray diffraction patterns, results show that different mechanical activation ways played different roles in structural changes of pyrite.
Similar content being viewed by others
References
Ahmadzadeh M, Ataie A, Mostafavi E. The Effects of Mechanical Activation Energy on the Solid-state Synthesis Process of BiFeO3[J]. Journal of Alloys and Compounds, 2015, 622: 548–556
Kristóf É, Juhász A Z. The Effect of Intensive Grinding on the Crystal Structure of Dolomite[J]. Powder Technology, 1993, 75: 145–152
Li H G, Zhao Z W, Zhao T C. Changes in Physical Chenmical Properties of Pyrite Mechanically Activated in a Vibration Mill[J]. Cent. South Univ. Technol., 1995, 26: 349–352
Li H G, Zhao Z W, Zhao T C, et al. Effect of Experimental Facility Style on the Activation of Mineral[J]. Cent. South Univ.Technol., 1997, 28: 130–133
Hu H P, Chen Q Y, Yin Z L, et al. Study on the Kinetics of Thermal Decomposition of Mechanically Activated Pyrites[J]. Thermochimica Acta, 2002, 389: 79–83
Eymery J, Yllif. Study of a Mechanochemical Transformation in Iron Pyrite[J]. Journal of Alloys and Compounds, 2000, 298:306-309
Warris C J, Mccormick P G. Mechanochemical Processing of Refractory yrite[J]. Minerals Engineering, 1997, 10: 1119–1125
Pornnpa K, Arthit N, Prinya C, et al. Thermal and Adhesive Properties of Epoxy Resin Cured with Cashew Nut Shell Liquid[J]. Thermochimica Acta, 2015, 600: 20–27
Huo S P, Wu G M, Chen J, et al. Curing Kinetics of Liginin and Cardanol Based Novolac Epoxy Resin with Methyl Tetrahydrophthalic Anhydride[J]. Thermochimica Acta, 2014, 587:18–23
Vyazovkin S, Burnham A K, Criado J M, et al. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data[J]. Thermochimica Acta, 2011, 520: 1–19
Darko M M, Sanja B O, Mladen B S, et al. Kinetics of Blackberry and Raspberry Seed Oils Oxidation by DSC[J]. Thermochimica Acta, 2015, 601: 39–44
Yolanda M, Lourdes F, Jordi P. Thermal Degradation Studies of oly (trimethylene carbonate) Blends with Either Polylactide or olycaprolactone [J]. Thermochimica Acta, 2012, 550: 65–75
Bagchi T P, Sen P K. Combined Differential and Integral Method for Analysis of Non-isothermal Kinetic Data[J]. Thermochimica Acta, 1981, 51:175–189
Parviz P, Eric F. Effects of Mechanical Activation on the Reduction Behavior of Hematite Concentrate[J]. Int. J. Miner. Process, 2007, 82: 96–105
Yang F Q, Wu C. Mechanism of Mechanical Activation for Spontaneous Combustion of Sulfide Minerals[J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 276–282
Wen F, Wang H, Tang Z X. Kinetic Study of the Redox Process of Iron Oxide for Hydrogen Production at Oxidation Step[J]. Thermochimica Acta, 2011, 520: 55–60
Zhao G L, Feng Y L, Wen Y H. Syntheses, Crystal Structures and Kinetic Mechanisms of Thermal Decomposition of Rare Earth Complexes with Schiff Base Derived from o-Vanillin and p-Toluidine [J]. Journal of Rare Earths, 2006, 24: 268–275
Jankovic B, Adnadevic B, Jovanovic J. Application of Model-fitting and Model-free Kinetics to the Study of Non-isothermal Dehydration of Equilibrium Swollen Poly(acrylic acid) Hydrogel: Thermogravimetric Analysis[J]. Thermo- chimica Thermochimica Acta, 2007, 452: 106–115
Yang F Q, Wu C, Shi Y. Thermal Properties of Sulfide Ores Determined by Using Thermo- gravimetric and Differential Scanning Calorimetric Method[J]. Science & Techology Review, 2009, 27: 66–71
Pu W F, Pang S S, Jia H. Using DSC/TG/DTA Techniques to Reevaluate the Effect of Clays on Crude Oil Oxidation Kinetics[J]. Journal of Petroleum Science and Engineering, 2015, 134:123–130
Sasidharan N, Hariharanath B, Rajendran A G. Thermal Decomposition Studies on Energetic Triazole Derivatives[J]. Thermochimica Acta, 2011, 520: 139–144
Xu B, Man J M, Wei C X. Methods for Determining Relative Crystallinity of Plant Starch X-ray Powder Diffraction Spectra[J]. Chinese Bulletin of Botany, 2012, 47: 278–285
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the National Natural Science Foundation of China (Nos.51174153 and 51374164) and Hubei Natural Science Foundation (No.2014CFB879)
Rights and permissions
About this article
Cite this article
Li, X., Chen, Z., Chen, X. et al. Effects of mechanical activation methods on thermo-oxidation behaviors of pyrite. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 30, 974–980 (2015). https://doi.org/10.1007/s11595-015-1260-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-015-1260-0