Abstract
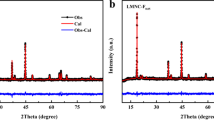
Mg-doped-LMR-NMC (Li1.2Ni0.15-xMgxMn0.55Co0.1 O2) is synthesized by combustion method followed by fluorine doping by solid-state synthesis. In this approach, we substituted the Ni2+ by Mg2+ in varying mole percentages (x = 0.02, 0.05, 0.08) and partly oxygen by fluorine (LiF: LMR-NMC = 1:50 wt%). The synergistic effect of both magnesium and fluorine substitution on electrochemical performance of LMR-NMC is studied by electrochemical impedance spectroscopy and galvanostatic-charge-discharge cycling. Mg-F-doped LMR-NMC (Mg 0.02 mol) composite cathodes shows excellent discharge capacity of ~300 mAh g−1 at C/20 rate whereas pristine LMR-NMC shows the initial capacity around 250 mAh g−1 in the voltage range between 2.5 and 4.7 V. Mg-F-doped LMR-NMC shows lesser Ohmic and charge transfer resistance, cycles well, and delivers a stable high capacity of ~280 mAh g−1 at C/10 rate. The voltage decay which is the major issue of LMR-NMC is minimized in Mg-F-doped LMR-NMC compared to pristine LMR-NMC.






Similar content being viewed by others
References
Armand M, Tarascon J-M (2008) Building better batteries. Nature 451:652–657
Scrosati B, Garche J (2010) Lithium batteries: status, prospects and future. J Power Sources 195:2419–2430
Wang C, John Appleby A, Little FE (2001) Charge–discharge stability of graphite anodes for lithium-ion batteries. J Electroanal Chem 497:33–46
Zhao L, Hu Y-S, Li H, Wang Z, Chen L (2011) Porous Li4Ti5O12 coated with N-doped carbon from ionic liquids for Li-ion batteries. Adv Mater 23:1385–1388
Yabuuchi N, Ohzuku T (2003) Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J Power Sources 119-121:171–174
Thackeray MM, Kang S-H, Johnson CS, Vaughey JT, Benedek R, Hackney SA (2007) Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater Chem 17:3112–3125
Martha SK, Nanda J, Veith GM, Dudney NJ (2012) Electrochemical and rate performance study of high-voltage lithium-rich composition: Li1.2Mn0.525Ni0.175Co0.1O2. J Power Sources 199:220–226
Mohanty D, Sefat AS, Kalnaus S, Li J, Meisner RA, Payzant EA, Abraham DP, Wood DL, Daniel C (2013) Investigating phase transformation in theLi1.2Co0.1Mn0.55Ni0.15O2 lithium-ion battery cathode during high-voltage hold (4.5 V) via magnetic, X-ray diffraction and electron microscopy studies. Journal of Material Chemistry A 1:6249–6261
Martha SK, Nanda J, Kim Y, Unocic RR, Pannala S, Dudney NJ (2013) Solid electrolyte coated high voltage layered–layered lithium-rich composite cathode: Li1.2Mn0.525Ni0.175Co0.1O2. Journal of Material Chemistry A 1:5587–5595
Martha SK, Nanda J, Veith GM, Dudney NJ (2012) Surface studies of high voltage lithium rich composition: Li1.2Mn0.525Ni0.175Co0.1O2. J Power Sources 216:179–186
Bettge M, Li Y, Gallagher K, Zhu Y, Wu Q, Lu W, Bloom I, Abraham DP (2013) Voltage fade of layered oxides: its measurement and impact on energy density. J Electrochem Soc 160(11):A2046–A2055
Nayak PK, Grinblat J, Levi E, Penki Ti R, Levi M, Sun Y-K, Markovsky B, Aurbach D (2016) Remarkably improved electrochemical performance of Li- and Mn-rich cathodes upon substitution of Mn with Ni. ACS Appl Mater Interfaces. doi:10.1021/acsami.6b07959
Armstrong AR, Holzapfel M, Novàk P, Johnson CS, Kang S-H, Thackeray MM, Bruce PG (2006) Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li [Ni0.2Li0.2Mn0.6] O2. J Am Chem Soc 128:8694–8698
Liu J, Manthiram A (2010) Functional surface modifications of a high capacity layered Li [Li0.2Mn0.54Ni0.13Co0.13] O2cathode. Journal of Material Chemistry 20:3961–3967
Chikkannanavar SB, Bernardi DM, Liu L (2014) A review of blended cathode materials for use in Li-ion batteries. J Power Sources 248:91–100
Fu LJ, Liu H, Li C, Wu YP, Rahm E, Holze R, Wu HQ (2006) Surface modifications of electrode materials for lithium ion batteries. Solid State Sci 8:113–128
Li C, Zhang HP, Fu LJ, Liu H, Wu YP, Rahm E, Holze R, Wu HQ (2006) Cathode materials modified by surface coating for lithium ion batteries. Electrochim Acta 51:3872–3883
Gallagher KG, Kang S-H, Park SU, Han SY (2011) xLi2MnO3·(1−x)LiMO2 blended with LiFePO4 to achieve high energy density and pulse power capability. J Power Sources 196:9702–9707
Park SH, Sun Y-K (2003) Synthesis and electrochemical properties of layered Li [Li0.15Ni(0.275−x/2)Al x Mn(0.575− x /2)] O2 materials prepared by sol–gel method. J Power Sources 119–121:161–165
Wang YX, Shang KH, He W, Ai XP, Cao YL, Yang HX (2015) Magnesium-doped Li1.2 [Co0.13Ni0.13Mn0.54] O2 for lithium-ion battery cathode with enhanced cycling stability and rate capability. ACS Applied Materials Interfaces 7:13014–13021
Sun YK, Jeon YS, Lee HJ (2000) Overcoming Jahn-Teller distortion for spinel Mn phase. Electrochem Solid-State Lett 3:7
Robert R, Villevieille C, Novák P (2014) Enhancement of the high potential specific charge in layered electrode materials for lithium-ion batteries. Journal of Material Chemistry A 2:8589–8598
Kim G-H, Kim J-H, Myung S-T, Yoon CS, Sun Y-K (2005) Improvement of high-voltage cycling behavior of surface-modified Li [Ni1/3Co1/3Mn1/3] O2 cathodes by fluorine substitution for Li-ion batteries. J Electrochem Soc 152:A1707–A1713
Shin HS, Park SH, Yoon CS, Sun YK (2005) Effect of fluorine on the electrochemical properties of layered LiNi0.43Co0.22Mn0.35O2 cathode materials via a carbonate process. Electrochem Solid-State Lett 8:A559
Woo SU, Park BC, Yoon CS, Myung ST, Prakash J, Sun YK (2007) Improvement of electrochemical performances of LiNi0.8Co0.1Mn0.1O2 cathode materials by fluorine substitution. J Electrochem Soc 154:A649
Shin HS, Shin D, Sun YK (2006) Improvement of electrochemical properties of Li [Ni0.4Co0.2Mn(0.4-x)Mgx] O2-yFy cathode materials at high voltage region. Electrochim Acta 52:1477
Kim G-H, Myung S-T, Bang HJ, Prakash J, Sun Y-K (2004) Synthesis and electrochemical properties of Li[Ni1/3Co1/3Mn(1/3-x)Mgx]O2-yFy via coprecipitation. Electrochem Solid-State Lett 7(12):A477–A480
Axelbaum RL, Lengyel M (2015) Doped lithium-rich layered composite cathode materials. United States Patent, US2015/0270545A1
Luo W, Zhou F, Zhao X, Lu Z, Li X, Dahn JR (2010) Synthesis, characterization, and thermal stability o LiNi1/3Mn1/3Co1/3−z Mg z O2,LiNi1/3−z Mn1/3Co1/3Mg z O2, and LiNi1/3Mn1/3−z Co1/3Mg z O2. Chem Mater 22:1164–1172
Mohanty D, Dahlberg K, King DM, David LA, Sefat AS, Wood DL, Daniel C, Dhar S, Mahajan V, Lee M, Albano F (2016) Modification of Ni-rich FCG NMC and NCA cathodes by atomic layer deposition: preventing surface phase transitions for high-voltage lithium-ion batteries. Nature Scientific Reports 6:26532. doi:10.1038/srep26532
Liu W, Pilgun O, Liu X, Lee M-J, Cho W, Chae S, Kim Y, Cho J (2015) Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew Chem 54:4440–4457
Acknowledgments
SKK acknowledges the University Grant Commision, SG acknowledges MHRD for fellowship, and SKM acknowledges DST-SERB (Grant no. SB/FT/CS-147/2014) for the financial support for this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishna Kumar, S., Ghosh, S. & Martha, S.K. Synergistic effect of magnesium and fluorine doping on the electrochemical performance of lithium-manganese rich (LMR)-based Ni-Mn-Co-oxide (NMC) cathodes for lithium-ion batteries. Ionics 23, 1655–1662 (2017). https://doi.org/10.1007/s11581-017-2018-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2018-9