Abstract
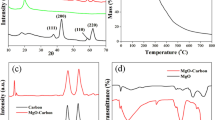
A sensitive and selective electrochemical Pb2+ sensor consisting of a gold-carbon foam/chitosan/gold (Au-CFs/Chit/Au)-modified electrode was prepared. The electrode was synthesized via an oil-in-water emulsion polymerization and carbonization approach. Phenolic resins were used as a carbon source. HAuCl4 was used as a gold source and as an acidic catalyst. Melamine was used as a coordination and coupling agent to control the size of the Au nanoparticles (AuNPs). The morphologies and microstructures of the Au-CFs were characterized using scanning electron microscopy, X-ray diffraction, and transmission electron microscopy. The results revealed that the carbon foams contained interconnected macropores with diameters of nearly 5.0 μm and AuNPs with mean diameters of approximately 20.0, 9.0, and 7.0 nm. Brunauer–Emmett–Teller analysis revealed that the biggest surface area is 653.82 m2/g for Au/CFs-7. The electrochemical properties of modified electrodes and their responses to Pb2+ were characterized using cyclic voltammetry and differential pulse anodic stripping voltammetry. The influence of the test conditions were studied to optimize operational parameters such as the choice of supporting electrolyte, pH, deposition potential, and deposition time. Under optimal conditions, typical Au/CFs-7-modified gold electrodes exhibited an excellent electrochemical response for Pb2+ with a wide linear response range from 0.01 to 1.2 μM, a correlation coefficient of 0.995, and a lower limit of detection of 0.63 nM with deposition time of 180 s (S/N = 3).






Similar content being viewed by others
References
Liu MC, Zhao GH, Tang YT, Yu ZM, Lei YZ, Li MF, Zhang YA, Li DM (2010) A simple, stable and picomole level lead sensor fabricated on DNA-based carbon hybridized TiO2 nanotube arrays. Environ Sci Technol 44:4241–4246
Nriagu JO, Pacyna JM (1988) A global assessment of natural sources of atmospheric trace metals. Nature 333:134–139
Zhang TJ, Li CY, Mao BJ, An Y (2015) Determination of Cd2+ by ultrasound-assisted square wave anodic stripping voltammetry with a boron-doped diamond electrode. Ionics 21:1761–1769
Rofouei M, Sabouri A, Ahmadalinezhad (2011) A solid phase extraction of ultra traces mercury (II) using octadecyl silica membrane disks modified by 1,3-bis(2-ethoxyphenyl)triazene (EPT) ligand and determination by cold vapor atomic absorption spectrometry. J Hazard Mater 192:1358–1363
Lee JM, Boyle EA, Echegoyen-Sanz Y, Fitzsimmons JN, Zhang RF, Kayser RA (2011) Analysis of trace metals (Cu, Cd, Pb, and Fe) in seawater using single batch nitrilotriacetate resin extraction and isotope dilution inductively coupled plasma mass spectrometry. Anal Chim Acta 686:93–101
Marguí E, Kregsamer P, Hidalgo M, Tapias J, Queralt I, Streli C (2010) Analytical approaches for Hg determination in wastewater samples by means of total reflection X-ray fluorescence spectrometry. Talanta 82:821–827
Zhao ZQ, Chen X, Yang Q, Liu JH, Huang XJ (2012) Beyond the selective adsorption of polypyrrole-reduced graphene oxide nanocomposite toward Hg2+: ultra-sensitive and -selective sensing Pb2+ by stripping voltammetry. Electrochem Commun 23:21–24
Wei Y, Gao C, Meng FL, Li HH, Wang L, Liu JH, Huang XJ (2012) SnO2/reduced graphene oxide nanocomposite for the simultaneous electrochemical detection of cadmium(II), lead(II), copper(II), and mercury(II): an interesting favorable mutual interference. J Phys Chem C 116:1034–1041
Dai XX, Qiu FG, Zhou X, Long YM, Li WF, Tu YF (2014) Amino-functionalized MCM-41 for the simultaneous electrochemical determination of trace lead and cadmium. Electrochim Acta 144:161–167
Chen XJ, Tian R, Zhang Q, Yao C (2014) Target-induced electronic switch for ultrasensitive detection of Pb2+ based on three dimensionally ordered macroporous Au–Pd bimetallic electrode. Biosens Bioelectron 53:90–98
Huang H, Chen T, Liu XY, Ma HY (2014) Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials. Anal Chim Acta 852:45–54
Rutyna SI, Korolczuk M (2014) Determination of lead and cadmium by anodic stripping voltammetry at bismuth film electrodes following double deposition and stripping steps. Sensors Actuators B 204:136–141
Li DY, Li J, Jia XF, Wang EK (2014) Gold nanoparticles decorated carbon fiber mat as a novel sensing platform for sensitive detection of Hg (II). Electrochem Commun 42:30–33
Xu XX, Duan GT, Li Y, Liu GQ, Wang JJ, Zhang HW, Dai ZF, Cai WQ (2014) Fabrication of gold nanoparticles by laser ablation in liquid and their application for simultaneous electrochemical detection of Cd2+, Pb2+, Cu2+, Hg2+. ACS Appl Mater Interfaces 6:65–71
Zhou L, Xiong W, Liu ST (2015) Preparation of a gold electrode modified with Au-TiO2 nanoparticles as an electrochemical sensor for the detection of mercury(II) ions. J Mater Sci 50:769–776
Dimitratos N, Lopez-Sanchez JA, Hutchings G (2012) Selective liquid phase oxidation with supported metal nanoparticles. J Chem Sci 3:20–44
Zhang JT, Jin ZY, Li WC, Dong W, Lu AH (2013) Graphene modified carbon nanosheets for electrochemical detection of Pb (II) in water. J Mater Chem A 1:13139–13145
Li J, Guo SJ, Zhai YM, Wang EK (2009) Nafion–graphene nanocomposite film as enhanced sensing platform for ultrasensitive determination of cadmium. Electrochem Commun 5(11):1085–1088
Wang LX, Bai J, Bo XJ, Zhang XL, Guo LP (2011) A novel glucose sensor based on ordered mesoporous carbon-Au nanoparticles nanocomposites. Talanta 83:1386–1391
Xiong W, Liu MX, Gan LH, Lv YK, Li Y, Yang L, Xu ZJ, Hao ZX, Liu HL, Chen LW (2011) A novel synthesis of mesoporous carbon microspheres for supercapacitor electrodes. J Power Sources 196:10461–10464
Xiong W, Liu MX, Gan LH, Lv YK, Xu ZJ, Hao ZX, Chen LW (2012) Preparation of nitrogen-doped macro-/mesoporous carbon foams as electrode material for supercapacitors. Colloids Surf A Physicochem Eng Asp 411:34–39
Datta KKR, Subba Reddy BV, Ariga K, Vinu A (2010) Gold nanoparticles embedded in a mesoporous carbon nitride stabilizer for highly efficient three-component coupling reaction. Angew Chem Int Ed 49:5961–5965
Yue W, Riehl BL, Pantelic N, Schluete KTR, Johnson JM, Wilson RA, Guo XF, King EE, Heineman WR (2012) Anodic stripping voltammetry of heavy metals on a metal catalyst free carbon nanotube electrode. Electroanalysis 24(5):1039–1046
Perone SP (1963) The application of stripping analysis to the determination of silver(I) using graphite. Electrodes Anal Chem 35:2091–2094
Xu RX, Yu XY, Gao C, Jing YJ, Han DD, Liu JH, Huang XJ (2013) Non-conductive nanomaterial enhanced electrochemical response in stripping voltammetry: the use of nanostructured magnesium silicate hollow spheres for heavy metal ions detection. Anal Chim Acta 790:31–38
Philips MF, Gopalan AI, Lee KP (2012) Development of a novel cyano group containing electrochemically deposited polymer film for ultrasensitive simultaneous detection of trace level cadmium and lead. J Hazard Mater 237–238:46–54
Wang ZM, Guo HW, Liu E, Yang GC, Khun NW (2010) Bismuth/polyaniline/glassy carbon electrodes prepared with different protocols for stripping voltammetric determination of trace Cd and Pb in solutions having surfactants. Electroanalysis 22:209–215
Zhu LD, Tian CY, Yang RL, Zhai JL (2008) Anodic stripping voltammetric determination of lead in tap water at an ordered mesoporous carbon/nafion composite film electrode. Electroanalysis 20:527–533
Mališića M, Janoševićb A, Šljukić Paunkovića B, Stojkovića I, Ćirić-Marjanovića G (2012) Exploration of MnO2/carbon composites and their application to simultaneous electroanalytical determination of Pb (II) and Cd (II). Electrochim Acta 74:158–164
Peng QM, Guo JX, Zhang QR, Xiang JY, Liu BZ, Zhou AG, Liu RP, Tian YJ (2014) Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J Am Chem Soc 136(11):4113–4116
Zhang QR, Du Q, Hua M, Jiao TF, Gao FM, Pan BC (2013) Sorption enhancement of lead ions from water by surface charged polystyrene-supported nano-zirconium oxide composites. Environ Sci Technol 47(12):6536–6544
Acknowledgments
The project was supported by the National Natural Science Foundation of China (No. 20873097, 21071113, 21471120), the Science Research Foundation of Wuhan Institute of Technology (K201450), and the Natural Science Foundation of Hubei Province (2014CFB782).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, L., Xiong, W. & Liu, S. Size-controlled growth of gold nanoparticle-doped carbon foams as sensitive electrochemical sensors for the determination of Pb(II). Ionics 22, 935–941 (2016). https://doi.org/10.1007/s11581-015-1608-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1608-7