Abstract
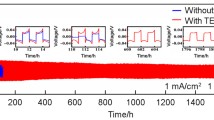
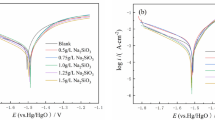
MoO3 was added to the zinc electrode of Zn/KOH/NiOOH cells. The combination of anodic polarization curve, constant current charge–discharge, and impedance spectra was used to investigate the effects of adding MoO3 on the electrochemical performance of zinc anode. The cell with MoO3 added in zinc anode reached 360 cycles before its capacity fell below 90 % of the initial capacity, while the cell without MoO3 failed after 180 cycles. Based on the scanning electron microscopy observation, the significant improvement of cycle stability can be attributed to the addition of MoO3, which helps zinc electrode to stay in a loose and porous structure, such texture is definitely in favor of the diffusion of OH− ions, facilitating the utilization of active material. But for standard electrode without MoO3, zinc anode is easy to passivate owing to the formation of dense ZnO film on zinc electrode surface. This compact film restricts OH− ion diffusion and decreases active area of zinc electrode. Therefore, MoO3-added electrode can effectively improve the cycle performance of the Ni–Zn battery.





Similar content being viewed by others
References
Kim H, Jeong G, Kim Y-U, Kim J-H, Park C-M, Sohn H-J (2013) Chem Soc Rev 42:9011
Li Y, Dai H (2014) Recent advances in zinc−air batteries. Chem Soc Rev 43:5257−5275
McBreen J (1994) J Power Sources 51:27
Wang K, Pei P, Ma Z, Xu H, Li P, Wang X (2014) Morphology control of zinc regeneration for zinc−air fuel cell and battery. J Power Sources 271:65−75
Simon Berners Hall and Jinrong Liu, compositions, zinc electrodes, batteries and their methods of manufacture. US. patent. US2011/0012055 A1.2011
Lee CW, Sathiyanarayanan K, Eom SW, Yun MS (2006) J Power Sources 160:1436
Wu JZ, Tu JP, Yuan YF et al (2009) J Alloys Compd 479:624
Yuan YF, Tu JP, Wu HM et al (2006) Electrochem Commun 8:653
Coates D, Ferreira E, Charkey A (1997) J Power Sources 65:109
Yu JX, Yang H, Ai XP, Zhu XM (2001) J Power Sources 103:93
McBreen J, Gannon E (1985) J Power Sources 15:169
Shivkumar R, Paruthimal Kalaignan G, Vasudevan T (1998) J Power Sources 75:90
Jain R, Adler TC, McLarnon FR et al (1992) J Appl Electrochem 22:1039
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
zhao, J., Zhou, D. & Gan, W. Effects of MoO3 on secondary alkaline zinc anode. Ionics 21, 1983–1988 (2015). https://doi.org/10.1007/s11581-015-1367-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1367-5