Abstract
The specific topological changes in dynamic functional networks and their role in cervical spondylotic myelopathy (CSM) brain function reorganization remain unclear. This study aimed to investigate the dynamic functional connection (dFC) of patients with CSM, focusing on the temporal characteristics of the functional connection state patterns and the variability of network topological organization. Eighty-eight patients with CSM and 77 healthy controls (HCs) were recruited for resting-state functional magnetic resonance imaging. We applied the sliding time window analysis method and K-means clustering analysis to capture the dFC variability patterns of the two groups. The graph-theoretical approach was used to investigate the variance in the topological organization of whole-brain functional networks. All participants showed four types of dynamic functional connection states. The mean dwell time in state 2 was significantly different between the two groups. Particularly, the mean dwell time in state 2 was significantly longer in the CSM group than in the healthy control group. Among the four states, switching of relative brain networks mainly included the executive control network (ECN), salience network (SN), default mode network (DMN), language network (LN), visual network (VN), auditory network (AN), precuneus network (PN), and sensorimotor network (SMN). Additionally, the topological properties of the dynamic network were variable in patients with CSM. Dynamic functional connection states may offer new insights into intrinsic functional activities in CSM brain networks. The variance of topological organization may suggest instability of the brain networks in patients with CSM.
Similar content being viewed by others
Introduction
Cervical spondylotic myelopathy (CSM) is the most common cause of cervical spinal cord compression, with impaired sensory and motor functions, which seriously affects the quality of life of patients (Yarbrough et al. 2012). Studies have indicated that the spinal cord has several connections with the brain structure and function, and that the high central nervous system can regulate the motor and sensory functions in patients with CSM (Bernabéu-Sanz et al. 2020; Dong et al. 2008; Duggal et al. 2010; Tinnermann et al. 2017). Brain plasticity or reorganization has been reported to affect neurological function recovery by affecting structural and functional changes in the cervical spinal cord (Duggal et al. 2010). Therefore, the underlying mechanism of central nervous system damage in patients with CSM may help explain the differences in surgical outcomes.
Previous neuroimaging studies have clarified impairments in the brain networks of patients with cervical spinal cord injury (Oni-Orisan et al. 2016; Ryan et al. 2018). Zhao et al. (Zhao et al. 2020) found cognitive deficits and their correlation with alterations in graph theory properties and functional connectivity in the sensorimotor network (SMN), default mode network (DMN), and visual network (VN). In addition, our previous study found alterations in the intrinsic functional plasticity within the SMN in patients with CSM (Zhou et al. 2015). Furthermore, we analyzed the disruption of human brain connectivity networks in patients with CSM using graph theory analysis (Cao et al. 2021). However, all these studies treated intrinsic brain activity as stable and invariable in whole-scan time courses, which is called static functional connectivity (sFC). SFC implicitly ignores the functional interaction over time by calculating the statistical correlation between regions during whole-scan time courses. Recently, emerging evidence has shown that functional connectivity between brain regions during scan sessions is highly dynamic (Allen et al. 2014; Damaraju et al. 2014; Di and Biswal 2015; Gonzalez-Castillo et al. 2014; Leonardi et al. 2013).
Many studies have pointed out that dynamic functional connectivity provides new insights for a better understanding of pathophysiological mechanisms in the central nervous system by capturing the transition between different states (Allen et al. 2014), (Li et al. 2020). Liao et al. (Liao et al. 2015) had demonstrated the characteristics of dynamic functional architecture in large-scale human brain networks during resting-state functional magnetic resonance imaging (rs-fMRI) to deepen the understanding of spontaneous human brain network dynamics. In addition, topological characteristics based on graph theory analysis have been regarded as a good way to explore the topological properties of human brain networks (Bullmore and Sporns 2009). Abnormalities of the brain networks were only partial representations of static functional connections, which inadequately represented the working patterns of brain networks in patients with CSM. As mentioned above, we explored sFC networks using the graph theory method; we also discuss the topological properties of dynamic functional connection networks.
Sliding window analysis is a method used for dynamic FC (dFC) analysis. The selection of the window length is a controversial issue (Shakil et al. 2016; Wilson et al. 2015). The most recommended window length is between 30 and 60 s (Li et al. 2018; Shakil et al. 2016). In addition, the K-means clustering method can divide these outcomes within all sliding time windows into several states to better describe the operating mode of the human brain during whole-scan time courses.
Accordingly, to capture the dynamic configurations of brain networks, we hypothesized that patients with CSM would exhibit dynamic interactions between brain networks. Our group examined (i) whether patients with CSM exhibit different patterns of dynamic connectomics compared to healthy controls (HCs); (ii) how the topological organization of brain endogenous functional activity changes on the time scale; and (iii) whether there is a potential relationship between the dFC variability alterations and the clinical characteristics of patients with CSM.
Materials and Methods
This study was approved by the Institutional Review Board of the First Affiliated Hospital of Nanchang University. All the patients’ consents were also required and obtained.
Participants
There were 88 patients with CSM (45 males and 43 females; mean age 49.22 ± 7.91 years; range 30–62 years) from the First Affiliated Hospital of Nanchang University and 77 HCs of level-matched age, sex, and education (39 males and 38 females; mean age 45.13 ± 8.76 years; range 25–64 years) were recruited in our study from January 2016 to December 2020. The gold diagnostic standard of CSM (Emery 2001): (1) clinical manifestations of cervical spinal cord injury; (2) radiographically confirmed spinal cord compression; (3) no amyotrophic lateral sclerosis, intramedullary tumors, secondary adhesion arachnoiditis, multiple peripheral neuritis, or spinal cord injury. In addition, patients were required to meet the following inclusion criteria: (1) volunteer to enroll in the study, (2) have clear evidence of cord compression on a cervical spine MRI, and (3) have had no medication therapy or decompression surgery. The exclusion criteria were as follows: (1) having other neurological disorders, (2) a history of psychiatric disorders, and (3) poor cooperation and so much head movement during image scanning. All patients were assessed with the Japanese Orthopaedic Association (JOA) scores (Azimi et al. 2012) and the Neck Disability Index (NDI) (Vernon 2008).
MRI data acquisition
All participants were scanned with a 3.0 T MRI (Siemens Trio Tim, Erlangen, Germany) scan with an 8-channel head coil. High-resolution anatomic images were acquired using a 3D T1-weighted spoiled gradient recall sequence with the following parameters: TR = 1900 ms, TE = 2.26 ms, flip angle = 9°, FOV = 256 × 256 mm, matrix = 256 × 256, slice thickness = 1 mm, number of slices = 176, voxel size = 1.0 × 1.0 × 1.0 mm3, and interslice gap = 0.5 mm. The echo-planar imaging (EPI) sequence parameters were as follows: TR/TE = 2000 ms / 30 ms, flip angle = 90°, FOV = 200 × 200 mm, matrix = 64 × 64, number of slices = 30, slice thickness = 4 mm, interslice gap = 1.2 mm, voxel size = 3.0 × 3.0 × 4.0 mm3, and 240 time points (8 min 6 s).
Data pre-processing
fMRI preprocessing was performed using the Data Processing Assistant for Resting-State (version 5.0; DPABI, http://www.restfmri.net) (Chao-Gan and Yu-Feng 2010). The procedures were as follows: (1) removal of the first ten time points; (2) slice timing correction; (3) head motion correction; (4) co-registration of functional data to the structural T1-weighted image; (5) normalization into the Montreal Neurological Institute (MNI) space with a resampling voxel size of 3 × 3 × 3 mm3; (6) removal of linear trends; (7) band-pass filtering (0.01–0.08 Hz); (8) nuisance covariate regression; (9) smoothing (6-mm full-width half-maximum Gaussian kernel).
Construction of dynamic functional networks
For each participant, we employed a commonly used sliding time window approach to estimate dFC based on the dynamic brain connectome (DynamicBC) analysis toolbox (Liao et al. 2014). An automated anatomical labeling 90 (AAL) atlas was used to divide the brain into 90 anatomical regions, with a sliding time window of 30 volumes (60 s). This window was shifted in time with a step size of 3 TR (6 s) (Li et al. 2018, 2019; Liao et al. 2018). The choice of window length was based on earlier studies that recommended a range of 30 to 60 s to capture spontaneous fluctuations (Luppi et al. 2021; Preti et al. 2017; Shakil et al. 2016). Pearson’s correlation coefficients were calculated between the time series from all brain regions, resulting in a 90 × 90 matrix per window per subject, with a total of 67 sliding time windows for each subject. Finally, we calculated the standard deviation of the Z value to represent the change in the time series of the correlation coefficient maps and characterize the variability of the dFC.
Characteristics of dynamic functional networks
K-means clustering analysis was used to conduct normalized graph clustering on the functional connection matrix (Shine et al. 2016). We then extracted the FC time-series signals to calculate the centroid, number of transitions, mean dwell time (MDT), and fractional windows. We then extracted the brain networks of all subjects in each state to further compare differences between groups within the same state using a two-sample t-test (with age, gender, and education as covariates; network-based statistics correction with edge p < 0.001, component p < 0.05, literation = 1000).
Graph theory analysis of dynamic functional networks
We extracted the dFC networks under all sliding time windows to further calculate their topological properties using graph-theory-based methods. Ultimately, 11,055 brain networks were identified. All network analyses were performed using the graph theoretical network analysis (GRETNA) toolbox 2.0.0 (http://www.nitrc.org/projects/gretna) (Wang et al. 2015). To generate an undirected and unweighted graph, the threshold range of sparsity was identified as 0.1–0.4, with an interval of 0.01 based on a previous study (Achard and Bullmore 2007). For the dFC network within each sliding time window, we calculated the variance of both the global and nodal network metrics (He et al. 2009; Koshimori et al. 2016; Yu et al. 2015). We then calculated the time-varying variance of the network metrics using a two-sample t-test to explore the topological properties of dynamic brain networks.
Statistical analysis
Statistical analyses were conducted using GraphPad software. Differences in categorical variables between groups were tested and compared using a chi-squared test, whereas those between continuous variables were evaluated using a two-sample t-test. We then assessed the relationships between the dynamic functional network metrics, JOA scores, and NDI scores in the CSM group.
Results
Demographics and clinical characteristics
The demographics and clinical characteristics of the subjects are shown in Table 1. Patients with CSM had a mean symptom duration of 10.24 ± 6.10 months, mean JOA score of 11.10 ± 1.78, and mean NDI score of 0.32 ± 0.09.
Temporal characteristics of dynamic functional networks
According to the above clustering criteria, we obtained the optimal number of clusters K = 4. We determined four types of states during resting-state MRI scans among subjects: state 1 (24.69%), state 2 (29.76%), state 3 (15.62%), and state 4 (29.92%) (Fig. 1). In particular, the MDT in state 2 was significantly longer in the CSM group than in the HC group (mean ± SD for healthy controls: 7.5 ± 13.6; for CSM patients: 13.4 ± 20.8, p < 0.05, Fig. 2). No group differences were observed in the number of transitions between the four states. We also performed a further analysis of correlations in the CSM group. However, there was no significant correlation between the dFC properties and clinical characteristics.
Differences of temporal properties between the two groups. No group differences were found in the number of transitions and state frequency between these four states. The mean dwell time of state 2 was significantly different (mean ± SD for healthy controls: 7.5 ± 13.6; for CSM patients: 13.4 ± 20.8, p < 0.05). Purple and blue represent the CSM and HC groups, respectively
Differences between CSM and HC groups within the same state
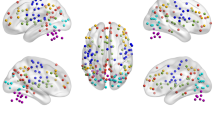
All the brain regions were anatomically divided into six modules (Fig. 3). Figure 4 shows a matrix diagram of the specific differences in the brain regions. Compared with HCs, CSM patients showed positive coupling between the SMN and language network (LN), whereas the salience network (SN) and precuneus network (PN) showed decreased functional connectivity in state 1. In state 2, compared with HCs, patients with CSM showed decreased functional connectivity in the DMN, whereas positive coupling existed in the executive control network (ECN) and SN. In state 3, compared with HCs, CSM patients showed negative coupling between the right DMN and SMN, whereas the LN and auditory network (AN) showed positive coupling. In state 4, compared with HCs, the VN and SMN showed negative coupling, but the ECN showed increased functional connectivity.
Connection patterns of state 1 (a), state 2 (b), state 3 (c) and state 4 (d). All brain regions were anatomically divided into six modules. Red represents the frontal lobe, green the prefrontal lobe, deep blue the subcortex, yellow the parietal lobe, purple the temporal lobe, and cyan the occipital lobe. The grey lines represent inter-module connectivities, and the rest of the lines represent intra-module connectivities
Topological properties of dynamic connectomics
We found that patients with CSM showed a higher variance in local efficiency in the right Heschel gyrus, left and right superior temporal gyrus (both belonging to AN), and left middle temporal gyrus (belonging to LN). In contrast, there were no significant differences in the variance of global efficiency. Table 2 and Fig. 5 show the significantly different brain regions of degree centrality, nodal efficiency, betweenness centrality, and nodal cluster coefficient between the two groups.
Discussion
In this study, we identified four different states by clustering all topography network maps across all sliding windows and subjects. We observed changes in the variability of network topological properties in dFC. Therefore, we discuss spontaneous dynamic reorganization in the whole brain of patients with CSM. Furthermore, we found that the variability in the network topological properties mainly focused on the DMN, LN, ECN, AN, and VN. In addition, we found that MDT in state 2 was significantly longer in the CSM group than in the HC group. According to previous studies, MDT means that subjects are primarily in the state of brain network connectivity patterns (Kim et al. 2017; Wang et al. 2019). This suggests that there is a longer dwell time pattern for state 2 in patients with CSM.
In state 1, patients with CSM showed positive coupling between the SMN and LN, whereas the SN and PN showed decreased functional connectivity. The SMN mainly regulates sensory and motor functions, and an abnormal connection of network functions corresponds to the clinical symptoms of patients. LN is important for maintaining sufficient language performance (Pistono et al. 2021).
As mentioned above, MDT in state 2 was significantly longer in the CSM group than in the HC group. This may indicate that patients with CSM are primarily in this state of brain function. The DMN and ECN are a pair of networks with opposite functions; they control cognitive and emotional executive functions to better internally self-focused attention of the central nervous system (Coppola et al. 2019; Davey et al. 2016; Qiao et al. 2020). The DMN supports internal psychological exploration and the ECN supports external stimuli and tasks. The switching of these two networks reflects the balance of conversion between internally and externally oriented cognition (Finn et al. 2015; Zou et al. 2021). We speculate that the increased functional connectivity of the ECN may be caused by the external stimulus of pain and stiffness in the neck in patients with CSM. The SN is mainly involved in attention, working memory, conflict monitoring, and salience detection (Alcauter et al. 2014; Steiner et al. 2020). The positive coupling between the ECN and the SN of patients with CSM may imply that external stimuli can enhance the coupling of these two networks within state 2. In this state, the brain mainly processes externally oriented cognition, attention, working memory, conflict monitoring, and salience detection.
In state 3, patients with CSM showed negative coupling between the DMN and SMN, whereas the LN and AN showed positive coupling. Prior studies have suggested that auditory expertise can enhance the perceptual categorization of speech (Bidelman 2015; Bidelman et al. 2014). Another recent study proposed that auditory experience modulates specific engagement and inter-regional communication between LNs and ANs (Bidelman and Walker 2019). Therefore, the positive coupling between LN and AN could promote the modulation of auditory and speech functions in patients with CSM.
In state 4, VN and SMN showed negative coupling, but ECN showed increased functional connectivity. VN plays a key role in the processing of visual movement, which can affect the acuity of visual space perception (Dole et al. 2013). Some researchers believe that the interaction between vision and sensorimotor function is crucial for movement control and motor learning (Glickstein 2000). Visual-sensorimotor interaction is important for movement control and motor learning (Greicius et al. 2003).
Compared to the HC group, the CSM group showed an increase in local efficiency. This may reflect abnormalities in local information transfer within the functional connectivity network in patients with CSM. In other words, the information transfer between critical nodes within a neighborhood tends to be more unstable and variable in patients with CSM. Some studies (Cohen 2018; Liao et al. 2014, 2017) have indicated that increased variability within dynamic brain networks may promote better integration of brain information and better flexibility of switching in cognitive processes. However, there were no significant differences in the global efficiency variance, which may indicate that information transfer in the whole brain is less affected, invariable, and remains stable. Besides, significant differences in variance also occurred in nodal efficiency, betweenness centrality, nodal degree, and nodal cluster coefficient. Interestingly, compared to HCs, variances in all nodal properties of time-varying brain connectivity were decreased in patients with CSM. These findings suggest that the brain areas of patients with CSM tend to be “fragile” and vulnerable to disturbance and damage, whereas the necessary resources may be recruited more quickly in the face of changing mission requirements in HCs. This finding is similar to the results of other neurological disorders, such as schizophrenia (Yu et al. 2015). For nodal efficiency, the significantly different brain regions were mainly located in the dorsal attention network, VN, ganglia network, AN, and ECN. For betweenness centrality, significantly different regions of the brain were mainly located in the DMN and LN. For nodal degree, significant differences in brain regions were mainly located in the ECN, DMN, dorsal attention network, and VN. For the nodal cluster coefficient, significantly different brain regions were mainly located in the AN. It was not difficult to observe that the brain regions with variance differences corresponded to the brain networks in the previous four states to some extent. Therefore, we have more sufficient reasons to believe that these networks play important roles in the pathogenesis of CSM.
This study had several limitations. First, it was a cross-sectional study that revealed patterns of dynamic connectivity of brain networks in patients with CSM. However, longitudinal studies are necessary to evaluate the effects of decompression surgery on alterations in the dynamic connectomics of brain networks. Second, this study included all patients with CSM. However, the dynamic brain network results in patients with mild, moderate, and severe CSM may differ. Finally, this study only analyzed the differences in the functional brain networks of patients with CSM. The topological properties of structural brain networks also require further exploration.
Conclusion
In this study, we explored the alterations in the dynamic topography of whole-brain functional networks in patients with CSM. We used the K-clustering method to separate the brain network patterns of patients with CSM into four states and concluded that patients with CSM are mainly in state 2. Therefore, we further speculate that the brain mainly processes externally oriented cognition, attention, working memory, conflict monitoring, and salience detection in patients with CSM. Our study not only found abnormalities in brain networks but also supplemented previous studies on resting-state brain networks in patients with CSM. Dynamic functional connection states may offer new insights into intrinsic functional activities in the brain networks of CSM. In addition, the variance of topological organization of whole-brain functional networks may suggest instability of the brain networks in patients with CSM.
Footnote
The authors are accountable for all aspects of the work and will ensure that any questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All figures and tables presented in this study are original and have not been previously published.
References
Achard S, Bullmore E (2007) Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3:e17
Alcauter S, Lin W, Smith J, Short S, Goldman B, Reznick J, Gilmore J, Gao W (2014) Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci 34:9067–9075
Allen E, Damaraju E, Plis S, Erhardt E, Eichele T, Calhoun V (2014) Tracking whole-brain connectivity dynamics in the resting state. Cerebral Cortex (New York, NY: 1991) 24:663–676
Azimi P, Mohammadi H, Montazeri A (2012) An outcome measure of functionality and pain in patients with lumbar disc herniation: a validation study of the Japanese Orthopedic Association (JOA) score. J Orthopaedi Sci 17:341–345
Bernabéu-Sanz Á, Mollá-Torró J, López-Celada S, Moreno López P, Fernández-Jover E (2020) MRI evidence of brain atrophy, white matter damage, and functional adaptive changes in patients with cervical spondylosis and prolonged spinal cord compression. Eur Radiol 30:357–369
Bidelman G (2015) Induced neural beta oscillations predict categorical speech perception abilities. Brain Lang 141:62–69
Bidelman G, Walker B (2019) Plasticity in auditory categorization is supported by differential engagement of the auditory-linguistic network. Neuroimage 201:116022
Bidelman G, Weiss M, Moreno S, Alain C (2014) Coordinated plasticity in brainstem and auditory cortex contributes to enhanced categorical speech perception in musicians. Eur J Neurosci 40:2662–2673
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198
Cao Y, Zhan Y, Du M, Zhao G, Liu Z, Zhou F, He L (2021) Disruption of human brain connectivity networks in patients with cervical spondylotic myelopathy. Quant Imaging Med Surg 11:3418–3430
Chao-Gan Y, Yu-Feng Z (2010) DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 4:13
Cohen J (2018) The behavioral and cognitive relevance of time-varying, dynamic changes in functional connectivity. Neuroimage 180:515–525
Coppola G, Di Renzo A, Petolicchio B, Tinelli E, Di Lorenzo C, Parisi V, Serrao M, Calistri V et al (2019) Aberrant interactions of cortical networks in chronic migraine: a resting-state fMRI study. Neurology 92:e2550–e2558
Damaraju E, Allen E, Belger A, Ford J, McEwen S, Mathalon D, Mueller B, Pearlson G et al (2014) Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage Clin 5:298–308
Davey C, Pujol J, Harrison B (2016) Mapping the self in the brain’s default mode network. Neuroimage 132:390–397
Di X, Biswal B (2015) Dynamic brain functional connectivity modulated by resting-state networks. Brain Struct Funct 220:37–46
Dole M, Meunier F, Hoen M (2013) Gray and white matter distribution in dyslexia: a VBM study of superior temporal gyrus asymmetry. PLoS ONE 8:e76823
Dong Y, Holly L, Albistegui-Dubois R, Yan X, Marehbian J, Newton J, Dobkin B (2008) Compensatory cerebral adaptations before and evolving changes after surgical decompression in cervical spondylotic myelopathy. J Neurosurg Spine 9:538–551
Duggal N, Rabin D, Bartha R, Barry R, Gati J, Kowalczyk I, Fink M (2010) Brain reorganization in patients with spinal cord compression evaluated using fMRI. Neurology 74:1048–1054
Emery S (2001) Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg 9:376–388
Finn E, Shen X, Scheinost D, Rosenberg M, Huang J, Chun M, Papademetris X, Constable R (2015) Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci 18:1664–1671
Glickstein M (2000) How are visual areas of the brain connected to motor areas for the sensory guidance of movement? Trends Neurosci 23:613–617
Gonzalez-Castillo J, Handwerker D, Robinson M, Hoy C, Buchanan L, Saad Z, Bandettini P (2014) The spatial structure of resting state connectivity stability on the scale of minutes. Front Neurosci 8:138
Greicius M, Krasnow B, Reiss A, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258
He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, Evans A (2009) Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain 132:3366–3379
Kim J, Criaud M, Cho S, Díez-Cirarda M, Mihaescu A, Coakeley S, Ghadery C, Valli M et al (2017) Abnormal intrinsic brain functional network dynamics in Parkinson’s disease. Brain 140:2955–2967
Koshimori Y, Cho S, Criaud M, Christopher L, Jacobs M, Ghadery C, Coakeley S, Harris M et al (2016) Disrupted nodal and hub organization account for brain network abnormalities in Parkinson’s disease. Front Aging Neurosci 8:259
Leonardi N, Richiardi J, Gschwind M, Simioni S, Annoni J, Schluep M, Vuilleumier P, Van De Ville D (2013) Principal components of functional connectivity: a new approach to study dynamic brain connectivity during rest. Neuroimage 83:937–950
Li J, Duan X, Cui Q, Chen H, Liao W (2019) More than just statics: temporal dynamics of intrinsic brain activity predicts the suicidal ideation in depressed patients. Psychol Med 49:852–860
Li L, Lu B, Yan C (2020) Stability of dynamic functional architecture differs between brain networks and states. Neuroimage 216:116230
Li R, Liao W, Yu Y, Chen H, Guo X, Tang Y, Chen H (2018) Differential patterns of dynamic functional connectivity variability of striato-cortical circuitry in children with benign epilepsy with centrotemporal spikes. Hum Brain Mapp 39:1207–1217
Liao W, Li J, Duan X, Cui Q, Chen H, Chen H (2018) Static and dynamic connectomics differentiate between depressed patients with and without suicidal ideation. Hum Brain Mapp 39:4105–4118
Liao W, Wu G, Xu Q, Ji G, Zhang Z, Zang Y, Lu G (2014) DynamicBC: a MATLAB toolbox for dynamic brain connectome analysis. Brain Connectiv 4:780–790
Liao X, Cao M, Xia M, He Y (2017) Individual differences and time-varying features of modular brain architecture. Neuroimage 152:94–107
Liao X, Yuan L, Zhao T, Dai Z, Shu N, Xia M, Yang Y, Evans A et al (2015) Spontaneous functional network dynamics and associated structural substrates in the human brain. Front Hum Neurosci 9:478
Luppi A, Golkowski D, Ranft A, Ilg R, Jordan D, Menon D, Stamatakis E (2021) Brain network integration dynamics are associated with loss and recovery of consciousness induced by sevoflurane. Hum Brain Mapp 42:2802–2822
Oni-Orisan A, Kaushal M, Li W, Leschke J, Ward B, Vedantam A, Kalinosky B, Budde M et al (2016) Alterations in cortical sensorimotor connectivity following complete cervical spinal cord injury: a prospective resting-state fMRI study. PLoS ONE 11:e0150351
Pistono A, Senoussi M, Guerrier L, Rafiq M, Giméno M, Péran P, Jucla M, Pariente J (2021) Language network connectivity increases in early Alzheimer’s disease. J Alzheimer’s Dis 82:447–460
Preti M, Bolton T, Van De Ville D (2017) The dynamic functional connectome: state-of-the-art and perspectives. Neuroimage 160:41–54
Qiao L, Luo X, Zhang L, Chen A, Li H, Qiu J (2020) Spontaneous brain state oscillation is associated with self-reported anxiety in a non-clinical sample. Sci Rep 10:19754
Ryan K, Goncalves S, Bartha R, Duggal N (2018) Motor network recovery in patients with chronic spinal cord compression: a longitudinal study following decompression surgery. J Neurosurg Spine 28:379–388
Shakil S, Lee C, Keilholz S (2016) Evaluation of sliding window correlation performance for characterizing dynamic functional connectivity and brain states. Neuroimage 133:111–128
Shine J, Bissett P, Bell P, Koyejo O, Balsters J, Gorgolewski K, Moodie C, Poldrack R (2016) The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron 92:544–554
Steiner L, Federspiel A, Slavova N, Wiest R, Grunt S, Steinlin M, Everts R (2020) Functional topography of the thalamo-cortical system during development and its relation to cognition. Neuroimage 223:117361
Tinnermann A, Geuter S, Sprenger C, Finsterbusch J, Büchel C (2017) Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science (new York, NY) 358:105–108
Vernon H (2008) The neck disability index: state-of-the-art, 1991–2008. J Manipul Physiol Ther 31:491–502
Wang J, Wang X, Xia M, Liao X, Evans A, He Y (2015) GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci 9:386
Wang X, Liao W, Han S, Li J, Zhang Y, Zhao J, Chen H (2019) Altered dynamic global signal topography in antipsychotic-naive adolescents with early-onset schizophrenia. Schizophr Res 208:308–316
Wilson R, Mayhew S, Rollings D, Goldstone A, Przezdzik I, Arvanitis T, Bagshaw A (2015) Influence of epoch length on measurement of dynamic functional connectivity in wakefulness and behavioural validation in sleep. Neuroimage 112:169–179
Yarbrough CK, Murphy RK, Ray WZ, Stewart TJ (2012) The natural history and clinical presentation of cervical spondylotic myelopathy. Adv Orthop 2012:480643
Yu Q, Erhardt E, Sui J, Du Y, He H, Hjelm D, Cetin M, Rachakonda S et al (2015) Assessing dynamic brain graphs of time-varying connectivity in fMRI data: application to healthy controls and patients with schizophrenia. Neuroimage 107:345–355
Zhao R, Su Q, Chen Z, Sun H, Liang M, Xue Y (2020) Neural correlates of cognitive dysfunctions in cervical spondylotic myelopathy patients: a resting-state fMRI study. Front Neurol 11:596795
Zhou F, Tan Y, Wu L, Zhuang Y, He L, Gong H (2015) Intrinsic functional plasticity of the sensory-motor network in patients with cervical spondylotic myelopathy. Sci Rep 5:9975
Zou Y, Tang W, Qiao X, Li J (2021) Aberrant modulations of static functional connectivity and dynamic functional network connectivity in chronic migraine. Quant Imaging Med Surg 11:2253–2264
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81460329) and the National Natural Science Foundation of Jiangxi Province (Nos. 20192ACBL20039 and 20181BAB205063).
Author information
Authors and Affiliations
Contributions
LH and GZ: (I) Conception and design. LH and FZ: (II) Administrative support. GZ, YZ, and JZ: (III) Provision of study materials or patients. GZ, YZ, and YC: (IV) Data collection and assembly. GZ and YZ: (V) Data analysis and interpretation. All authors: (VI) Manuscript writing. All authors: (VII) Final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest. The authors have no relevant financial or non-financial interests to disclose. The authors have no financial or proprietary interest in any material discussed in this article.
Ethical approval
This study was approved by the institutional review board of the First Affiliated Hospital of Nanchang University. This study was conducted per the ethical principles for medical research involving human subjects, as stipulated in the Declaration of Helsinki of the World Medical Association. Written informed consent was obtained from each participant before the study.
Data availability
The data that support the findings of this study are available from the First Affiliated Hospital of Nanchang University, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. However, the data are available from the authors upon reasonable request and with permission from the First Affiliated Hospital of Nanchang University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, G., Zhan, Y., Zha, J. et al. Abnormal intrinsic brain functional network dynamics in patients with cervical spondylotic myelopathy. Cogn Neurodyn 17, 1201–1211 (2023). https://doi.org/10.1007/s11571-022-09807-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-022-09807-0