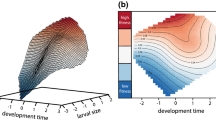
Abstract
Many pelagic fish species have a life history that involves producing a large number of small eggs. This is the result of a trade-off between fecundity and larval survival probability. There are also trade-offs involving other traits, such as larval swimming speed. Swimming faster increases the average food encounter rate but also increases the metabolic cost. Here we introduce an evolutionary model comprising fecundity and swimming speed as heritable traits. We show that there can be two evolutionary stable strategies. In environments where there is little noise in the food encounter rate, the stable strategy is a low-fecundity strategy with a swimming speed that minimises the mean time taken to reach reproductive maturity. However, in noisy environments, for example where the prey distribution is patchy or the water is turbulent, strategies that optimise mean outcomes are often outperformed by strategies that increase inter-individual variance. We show that, when larval growth rates are unpredictable, a high-fecundity strategy is evolutionarily stable. In a population following this strategy, the swimming speed is higher than would be anticipated by maximising the mean growth rate.



Similar content being viewed by others
References
Andersen KH, Beyer J, Pedersen M, Andersen NG, Gislason H (2008) Life-history constraints on the success of the many small eggs reproductive strategy. Theor Popul Biol 73(4):490–497
Benoît E, Rochet M-J (2004) A continuous model of biomass size spectra governed by predation and the effects of fishing on them. J Theor Biol 226(1):9–21
Burrow JF, Horwood JW, Pitchford JW (2011) The importance of variable timing and abundance of prey for fish larval recruitment. J Plankton Res 33:1153–1162
Chambers RC, Leggett WC (1996) Maternal influences on variation in egg sizes in temperate marine fishes. Am Zool 36(2):180–196
Chambers RC, Waiwood KG (1996) Maternal and seasonal differences in egg sizes and spawning characteristics of captive Atlantic cod, Gadus morhua. Can J Fish Aquat Sci 53(9):1986–2003
Chick JH, Van Den Avyle MJ (2000) Effects of feeding ration on larval swimming speed and responsiveness to predator attacks: implications for cohort survival. Can J Fish Aquat Sci 57(1):106–115
China V, Holzman R (2014) Hydrodynamic starvation in first-feeding larval fishes. Proc Natl Acad Sci 111(22):8083–8088
Currey JD, Pitchford JW, Baxter PD (2007) Variability of the mechanical properties of bone, and its evolutionary consequences. J R Soc Interface 4(12):127–135
Darowski K, Takashima F, Law Y (1988) Bioenergetic model of planktivorous fish feeding, growth and metabolism: theoretical optimum swimming speed of fish larvae. J Fish Biol 32(3):443–458
Duarte CM, Alcaraz M (1989) To produce many small or few large eggs: a size-independent reproductive tactic of fish. Oecologia 80(3):401–404
Elgar MA (1990) Evolutionary compromise between a few large and many small eggs: comparative evidence in teleost fish. Oikos 59:283–287
Fisher R, Bellwood DR (2003) Undisturbed swimming behaviour and nocturnal activity of coral reef fish larvae. Mar Ecol Prog Ser 263:177–188
Fisher R, Leis JM (2010) Swimming speeds in larval fishes: from escaping predators to the potential for long distance migration. In: Domenici P, Kapoor BG (eds) Fish locomotions: an eco-ethological perspective, chap 11. CRC Press, pp 333–373
Fisher R, Leis JM, Clark DL, Wilson SK (2005) Critical swimming speeds of late-stage coral reef fish larvae: variation within species, among species and between locations. Mar Biol 147(5):1201–1212
Fisher R, Sogard SM, Berkeley SA (2007) Trade-offs between size and energy reserves reflect alternative strategies for optimizing larval survival potential in rockfish. Mar Ecol Prog Ser 344:257–270
Grimmett G, Stirzaker D (1992) Probability and random processes. Oxford University Press, Oxford
Houde ED (1997) Patterns and consequences of selective processes in teleost early life histories. In: Chambers RC, Trippel EA (eds) Early life history and recruitment in fish populations, chap 6. Springer, Netherlands, pp 173–196
James A, Pitchford JW, Brindley J (2003) The relationship between plankton blooms, the hatching of fish larvae, and recruitment. Ecol Modell 160(1):77–90
Jennings S, Warr KJ (2003) Environmental correlates of large-scale spatial variation in the \(\delta \)15N of marine animals. Mar Biol 142(6):1131–1140
Law R, Plank MJ, Kolding J (2015) Balanced exploitation and coexistence of interacting, size-structured, fish species. Fish Fish. doi:10.1111/faf.12098
Levitan DR (1993) The importance of sperm limitation to the evolution of egg size in marine invertebrates. Am Nat 141:517–536
MacKenzie BR, Kiørboe T (1995) Encounter rates and swimming behavior of pause-travel and cruise larval fish predators in calm and turbulent laboratory environments. Limnol Oceanogr 40(7):1278–1289
Maynard-Smith J (1982) Evolution and the theory of games. Cambridge University Press, Cambridge
Mendiola D, Alvarez P, Cotano U, Etxebeste E, de Murguia AM (2006) Effects of temperature on development and mortality of atlantic mackerel fish eggs. Fish Res 80(2):158–168
Pitchford JW, Brindley J (2001) Prey patchiness, predator survival and fish recruitment. Bull Math Biol 63(3):527–546
Pitchford JW, James A, Brindley J (2003) Optimal foraging in patchy turbulent environments. Mar Ecol Prog Ser 256:99–110
Pitchford JW, James A, Brindley J (2005) Quantifying the effects of individual and environmental variability in fish recruitment. Fish Oceanogr 14(2):156–160
Rijnsdorp AD, Ibelings B (1989) Sexual dimorphism in the energetics of reproduction and growth of North Sea plaice, Pleuronectes platessa L. J Fish Biol 35(3):401–415
Tsuda A, Sugisaki H, Ishimaru T, Saino T, Sato T (1993) White-noise-like distribution of the oceanic copepod Neocalanus cristatus in the subarctic North Pacific. Mar Ecol Prog Ser 97:39–46
Ware DM, Lambert TC (1985) Early life history of atlantic mackerel (Scomber scombrus) in the southern Gulf of St. Lawrence. Can J Fish Aquat Sci 42(3):577–592
Winemiller KO, Rose KA (1993) Why do most fish produce so many tiny offspring? Am Nat 142:585–603
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
We define dimensionless variables
where \(t_\mathrm {ref}=4cx_m/(b^2x_p^2)\) and \(v_\mathrm {det}= bx_p/(2c)\), which is the swimming speed that maximises the expected growth rate, i.e. the deterministic optimum. Then Eq. (1) becomes
where
and \(\hat{W} = W/t_\mathrm {ref}^{1/2}\) is a standard Brownian motion with respect to dimensionless time \(\hat{t}\). The initial condition in the new variables is \(\hat{X}(0)=x_0/x_m\), and the fish reaches maturity when \(\hat{X}=1\). Dropping the hats, this is equivalent to Eq. (1) with the growth rate and diffusivity given in Eqs. (2) and (3).
Rights and permissions
About this article
Cite this article
Plank, M.J., Pitchford, J.W. & James, A. Evolutionarily Stable Strategies for Fecundity and Swimming Speed of Fish. Bull Math Biol 78, 280–292 (2016). https://doi.org/10.1007/s11538-016-0143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-016-0143-7