Abstract
Background
Targeted therapies (TTs) have revolutionised cancer treatment with their enhanced specificity of action. Compared with conventional therapies, TTs are delivered over a longer period and often have unusual symptom profiles. Patient-reported outcome measures such as symptom side-effect lists need to be developed in a time-efficient manner to enable a rapid and full evaluation of new treatments and effective clinical management
Objective
The aim of this study was to develop a set of TT-related symptoms and identify the optimal method for developing symptom lists.
Patients and Methods
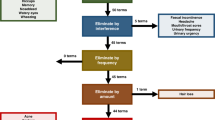
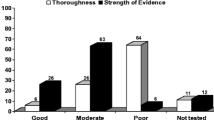
Symptoms from TT treatment in the context of Chronic Myeloid Leukaemia (CML), HER2-positive breast cancer, or Gastrointestinal Stromal Tumours (GIST) were identified through literature reviews, interviews with healthcare professionals (HCPs) and patients, and patient focus groups. The symptom set was then pilot tested in patients across the three cancer diagnoses: The number of items derived from each source (literature, patients, or HCPs) were compared.
Results
A total of 316 patients and 86 HCPs from 16 countries participated. An initial set of 209 symptoms was reduced to 61 covering 12 symptom categories. Patient interviews made the greatest contribution to the item set.
Conclusions
Symptom lists should be created based on input from patients. The item set described will be applicable to the assessment of new TTs, and in monitoring treatment.

Similar content being viewed by others
References
US Food and Drug Administration. Guidance for industry: patient-reported outcome measures—use in medical product development to support labeling claims
Basch E. Patient-reported outcomes—harnessing patients’ voices to improve clinical care. N Engl J Med. 2017;376(2):105–8.
Kluetz PG, Slagle A, Papadopoulos EJ, Johnson LL, Donoghue M, Kwitkowski VE, et al. Focusing on core patient-reported outcomes in cancer clinical trials: symptomatic adverse events, physical function, and disease-related symptoms. Clin Cancer Res. 2016;22(7):1553–8.
European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man. The use of patient-reported outcome (PRO) measures in oncology studies. Canary Wharf, London. EMA; 2016. https://www.ema.europa.eu/en/appendix-2-guideline-evaluation-anticancer-medicinal-products-man-use-patient-reported-outcome-pro
Groenvold M, Aaronson NK, Darlington ASE, Fitzsimmons D, Greimel E, Holzner B, et al. Focusing on core patient-reported outcomes in cancer clinical trials—letter. Clin Cancer Res. 2016;22(22):5617.
Bottomley A, Reijneveld JC, Koller M, Flechtner H, Tomaszewski KA, Greimel E, et al. Current state of quality of life and patient-reported outcomes research. Eur J Cancer. 2019;121:55–63.
Judson TJ, Bennett AV, Rogak LJ, Sit L, Barz A, Kris MG, et al. Feasibility of long-term patient self-reporting of toxicities from home via the Internet during routine chemotherapy. J Clin Oncol. 2013;31(20):2580–5.
Warrington L, Absolom K, Holch P, Gibson A, Clayton B, Velikova G. Online tool for monitoring adverse events in patients with cancer during treatment (eRAPID): field testing in a clinical setting. BMJ Open. 2019;9(1):e025185.
Snyder CF, Blackford AL, Wolff AC, Carducci MA, Herman JM, Wu AW, et al. Feasibility and value of Patient Viewpoint: a web system for patient-reported outcomes assessment in clinical practice. Psychooncology. 2013;22(4):895–901.
Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–65.
National Cancer Institute. Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE™). https://outcomes.cancer.gov/tools/pro-ctcae.html.
Petersen MA, Aaronson NK, Arraras JI, Chie W-C, Conroy T, Costantini A, et al. The EORTC CAT core—the computer adaptive version of the EORTC QLQ-C30 questionnaire. Eur J Cancer. 2018;100:8–16.
Cella DGR, Lai JS, Choi S. The future of outcomes measurement: item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res. 2007;16(Suppl 1):133–41.
de Wit M, Hajos T. Health-related quality of life. In: Gellman MD, Turner JR, editors. Encyclopedia of behavioral medicine. New York: Springer; 2013. p. 929–31.
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. First-line Herceptin monotherapy in metastatic breast cancer. Oncology. 2001;61(Suppl 2):37–42.
Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344(14):1038–42.
Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344(14):1052–6.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80.
Sodergren SC, White A, Efficace F, Sprangers M, Fitzsimmons D, Bottomley A, et al. Systematic review of the side effects associated with tyrosine kinase inhibitors used in the treatment of gastrointestinal stromal tumours on behalf of the EORTC Quality of Life Group. Critic Rev Oncol Hematol. 2014;91(1):35–46.
Guérin A, Chen L, Ionescu-Ittu R, Marynchenko M, Nitulescu R, Hiscock R, et al. Impact of low-grade adverse events on health-related quality of life in adult patients receiving imatinib or nilotinib for newly diagnosed Philadelphia chromosome positive chronic myelogenous leukemia in chronic phase. Curr Med Res Opin. 2014;30(11):2317–28.
Efficace F, Rosti G, Aaronson N, Cottone F, Angelucci E, Molica S, et al. Patient- versus physician reporting of symptoms and health status in chronic myeloid leukemia. Haematologica. 2014;99(4):788–93.
Johnson CD, Aaronson N, Blazeby J et al. EORTC Quality of Life Group: guidelines for developing questionnaire modules. Brussels: EORTC Quality of Life Group; 2011.
Kuliś D BA, Whittaker C, van de Poll-Franse LV, Darlington A, Holzner B, Koller M, Reijneveld JC, Tomaszewski K, Grønvold M, on behalf of the EORTC Quality of Life Group, editor The Use of the EORTC Item Library to Supplement EORTC Quality of Life Instruments. ISPOR 20th Annual European Congress. Glasgow: Value in Health. 2017.
Vachalec SBK, Bottomley A, Blazeby J, Flechtner H, Ruyskart P. EORTC item bank guidelines. Brussels: EORTC Publications; 2001.
Mouillet G, Fritzsch J, Paget-Bailly S, Pozet A, Es-Saad I, Meurisse A, et al. Health-related quality of life assessment for patients with advanced or metastatic renal cell carcinoma treated with a tyrosine kinase inhibitor using electronic patient-reported outcomes in daily clinical practice (QUANARIE trial): study protocol. Health Quality Life Outcomes. 2019;17(1):25.
Bell JA, Galaznik A, Pompilus F, Strzok S, Bejar R, Scipione F, et al. A pragmatic patient-reported outcome strategy for rare disease clinical trials: application of the EORTC item library to myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Patient Rep Outcomes. 2019;3(1):35.
Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851–7.
Efficace F, Baccarani M, Breccia M, Saussele S, Abel G, Caocci G, et al. International development of an EORTC questionnaire for assessing health-related quality of life in chronic myeloid leukemia patients: the EORTC QLQ-CML24. Qual Life Res. 2014;23(3):825–36.
Milanezi FCS, Schmitt FC. EGFR/HER2 in breast cancer: a biological approach for molecular diagnosis and therapy. Expert Rev Mol Diagn. 2008;8:417–34.
Baar J. Biological therapy of breast cancer: recent clinical applications. Curr Opin Investig Drugs. 2007;8:987–95.
Verweij JCP, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–34.
Springfield (MA). Merriam-Webster I. Webster's third new international dictionary of the English language, unabridged. 2002.
Sodergren SC, Copson E, White A, Efficace F, Sprangers M, Fitzsimmons D, et al. Systematic review of the side effects associated with anti-HER2-targeted therapies used in the treatment of breast cancer, on behalf of the EORTC quality of life group. Target Oncol. 2016;11(3):277–92.
Efficace F, Cocks K, Breccia M, Sprangers M, Meyers CA, Vignetti M, et al. Time for a new era in the evaluation of targeted therapies for patients with chronic myeloid leukemia: inclusion of quality of life and other patient-reported outcomes. Crit Rev Oncol Hematol. 2012;81(2):123–35.
Bjelic-Radisic V, Cardoso F, Cameron D, Brain E, Kuljanic K, da Costa RA, et al. An international update of the EORTC questionnaire for assessing quality of life in breast cancer patients: EORTC QLQ-BR45. Ann Oncol. 2020;31(2):283–8.
Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22(4):714–24.
Kluetz PG, Chingos DT, Basch EM, Mitchell SA. Patient-reported outcomes in cancer clinical trials: measuring symptomatic adverse events with the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Am Soc Clin Oncol Educ Book. 2016;36:67–73.
Acknowledgements
We would like to express our gratitude to the patients and healthcare professionals who took part in this research project and shared their views and experiences. We would also like to acknowledge the support of staff at the collaborating centres.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
Funding for this work was awarded by the EORTC Quality of Life Group (QLG) (Reference Number 010/2010). The QLG awarded funding in a competitive peer-reviewed grant application process. The executive Committee of the QLG reviewed and approved the final manuscript before submission for consideration, ‘on behalf of the EORTC QLG’.
Conflict of interest
Juan Ignacio Arraras received funding from “la Caixa” Foundation (ID 1000010434), Caja Navarra Foundation and UNED Pamplona, under agreement LCF/PR/PR15/51100007. The remaining authors declare that they have no conflicts of interest that might be relevant to the contents of this article.
Ethics approval
Ethical approval for this study was awarded for the Lead site (NRES Committee South Central Southampton B 11/SC/0412). Each collaborating centre also received their local approvals.
Consent to participate
All participants gave their informed consent to participate and for their anonymous data to be shared.
Consent for publication
All authors and the EORTC QLG granted their permission for the publication of this article.
Data availability
Raw data generated from this work cannot be shared as permissions were not requested from participants. The symptom set generated from this work is available from the EORTC QLG upon request at https://qol.eortc.org/questionnaires/.
Code availability
Not applicable.
Author contributions
All authors contributed to the manuscript preparation, editing and review.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sodergren, S.C., Wheelwright, S.J., Fitzsimmons, D. et al. Developing Symptom Lists for People with Cancer Treated with Targeted Therapies. Targ Oncol 16, 95–107 (2021). https://doi.org/10.1007/s11523-020-00769-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00769-z