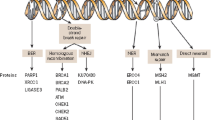
Abstract
Developments in systemic therapies beyond traditional cytotoxic chemotherapy have resulted in an unparalleled number of US Food and Drug Administration approvals in the past 5 years for ovarian, endometrial, and cervical cancer. In this review, we highlight registration trials leading to recent Food and Drug Administration approvals for targeted systemic therapies in advanced gynecologic malignancies, encompassing three classes of agents: the antiangiogenic anti-vascular endothelial growth factor antibody bevacizumab in ovarian and cervical cancer, poly (ADP-ribose) polymerase inhibitors in ovarian cancer, and the immune checkpoint inhibitor pembrolizumab in cervical and endometrial cancer. The addition of bevacizumab to chemotherapy has been approved in frontline and relapsed advanced ovarian cancer, in both platinum-resistant and platinum-sensitive settings. Three poly (ADP-ribose) polymerase inhibitors are approved for women with ovarian cancer. Olaparib and rucaparib are utilized in recurrent germline or somatic BRCA mutated ovarian cancer. Along with a third poly (ADP-ribose) polymerase inhibitor, niraparib, they are also Food and Drug Administration approved as maintenance therapy regardless of BRCA mutation status for patients with recurrent ovarian cancer in complete or partial response to platinum-based chemotherapy. More recently, olaparib was approved as maintenance therapy for BRCA mutated ovarian cancer following first-line platinum-based chemotherapy. Pembrolizumab has been approved for relapsed cervical cancer with programmed death ligand 1 positivity and relapsed solid tumors with mismatch repair deficiency, which applies to 30% of endometrial cancers. Together, these therapies represent the advent of personalized medicine in gynecologic malignancies. Additional information is required to determine cost-effective strategies for incorporating these therapies and rational means of sequencing these agents.
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Suh DH, Kim M, Kim K, Kim HJ, Lee KH, Kim JW. Major clinical research advances in gynecologic cancer in 2016: 10-year special edition. J Gynecol Oncol. 2017;28(3):e45. https://doi.org/10.3802/jgo.2017.28.e45.
Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–43. https://doi.org/10.1056/NEJMoa1309748.
Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. https://doi.org/10.1200/jco.2013.51.4489.
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–50. https://doi.org/10.1200/jco.2014.56.2728.
Homsi J, Daud AI. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control. 2007;14(3):285–94. https://doi.org/10.1177/107327480701400312.
Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–45. https://doi.org/10.1200/jco.2012.42.0505.
Aghajanian C, Goff B, Nycum LR, Wang YV, Husain A, Blank SV. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. 2015;139(1):10–6. https://doi.org/10.1016/j.ygyno.2015.08.004.
Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group Study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(6):779–91. https://doi.org/10.1016/s1470-2045(17)30279-6.
Coleman RL, Enserro D, Spirtos N, Herzog TJ, Sabbatini P, Armstrong DK, et al. A phase III randomized controlled trial of secondary surgical cytoreduction (SSC) followed by platinum-based combination chemotherapy (PBC), with or without bevacizumab (B) in platinum-sensitive, recurrent ovarian cancer (PSOC): a NRG Oncology/Gynecologic Oncology Group (GOG) study. J Clin Oncol. 2018;36(15_Suppl.):5501. https://doi.org/10.1200/jco.2018.36.15_suppl.5501.
F. Hoffman-La Roche Ltd. Roche medicine Avastin receives EU approval for the treatment of women with newly diagnosed, advanced ovarian cancer. https://www.roche.com/media/releases/med-cor-2011-12-23.htm. Accessed 7 Nov 2018.
Chugai Pharmceutical Co. Ltd. Anti-cancer agent “Avastin®,” obtained approval for additional indication of ovarian Cancer. https://www.chugai-pharm.co.jp/news/cont_file_dl.php?f=131122eAvastin_OC_Approval.pdf&src=[%0],[%1]&rep=130,260. Accessed 20 Apr 2019.
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. https://doi.org/10.1056/NEJMoa1104390.
Roche Investor Update. FDA approves Roche’s Avastin (bevacizumab) plus chemotherapy as a treatment for women with advanced ovarian cancer following initial surgery. https://www.roche.com/investors/updates/inv-update-2018-06-13.htm. Accessed 20 Jun 2018.
Gee ME, Faraahi Z, McCormick A, Edmondson RJ. DNA damage repair in ovarian cancer: unlocking the heterogeneity. J Ovarian Res. 2018;11(1):50. https://doi.org/10.1186/s13048-018-0424-x.
Chen Y, Du H. The promising PARP inhibitors in ovarian cancer therapy: from olaparib to others. Biomed Pharmacother. 2018;99:552–60. https://doi.org/10.1016/j.biopha.2018.01.094.
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. https://doi.org/10.1056/NEJMoa0900212.
Merck Newsroom. Lynparza® (olaparib) tablets receive EU approval for the treatment of platinum-sensitive relapsed ovarian cancer. https://www.mrknewsroom.com/news-release/oncology/lynparza-olaparib-tablets-receive-eu-approval-treatment-platinum-sensitive-rel. Accessed 20 Apr 2019.
AstraZeneca. Lynparza receives approval in Japan for the treatment of advanced ovarian cancer. https://www.astrazeneca.com/media-centre/press-releases/2018/lynparza-receives-approval-in-japan-for-the-treatment-of-advanced-ovarian-cancer-19012018.html. Accessed 20 Apr 2019.
AstraZeneca. Lynparza™ approved in the European Union as first-in-class treatment for advanced BRCA-mutated ovarian cancer. https://www.astrazeneca.com/media-centre/press-releases/2014/lynparza-approved-european-union-brca-mutated-ovarian-cancer-treatment-18122014.html#. Accessed 20 Apr 2019.
Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–84. https://doi.org/10.1016/s1470-2045(17)30469-2.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92. https://doi.org/10.1056/NEJMoa1105535.
Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–505. https://doi.org/10.1056/NEJMoa1810858.
Clovis Oncology. Clovis Oncology announces European Commission authorization of Rubraca® (rucaparib) for women with recurrent ovarian cancer. https://ir.clovisoncology.com/investors-and-news/news-releases/press-release-details/2018/Clovis-Oncology-Announces-European-Commission-Authorization-of-Rubraca-rucaparib-for-Women-with-Recurrent-Ovarian-Cancer/default.aspx. Accessed 20 Apr 2019.
Kristeleit R, Shapiro GI, Burris HA, Oza AM, LoRusso P, Patel MR, et al. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23(15):4095–106. https://doi.org/10.1158/1078-0432.CCR-16-2796.
Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75–87. https://doi.org/10.1016/S1470-2045(16)30559-9.
Clovis Oncology. Clovis Oncology announces European Commission authorization of Rubraca® (rucaparib) tablets as maintenance treatment for women with relapsed ovarian cancer. https://ir.clovisoncology.com/investors-and-news/news-releases/press-release-details/2019/Clovis-Oncology-Announces-European-Commission-Authorization-of-Rubraca-rucaparib-Tablets-as-Maintenance-Treatment-for-Women-with-Relapsed-Ovarian-Cancer/default.aspx. Accessed 20 Apr 2019.
Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949–61. https://doi.org/10.1016/s0140-6736(17)32440-6.
Tesaro. Tesaro announces European Commission approval of Zejula® for women with recurrent ovarian cancer. Available from: http://ir.tesarobio.com/news-releases/news-release-details/tesaro-announces-european-commission-approval-zejular-women. Accessed 20 Apr 2019.
Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–64. https://doi.org/10.1056/NEJMoa1611310.
Gene Inc. Avastin prescribing information. 2018. https://www.gene.com/download/pdf/avastin_prescribing.pdf. Accessed 9 Dec 2018.
Cohn DE, Kim KH, Resnick KE, O’Malley DM, Straughn JM Jr. At what cost does a potential survival advantage of bevacizumab make sense for the primary treatment of ovarian cancer? A cost-effectiveness analysis. J Clin Oncol. 2011;29(10):1247–51. https://doi.org/10.1200/JCO.2010.32.1075.
Mehta DA, Hay JW. Cost-effectiveness of adding bevacizumab to first line therapy for patients with advanced ovarian cancer. Gynecol Oncol. 2014;132(3):677–83. https://doi.org/10.1016/j.ygyno.2014.01.021.
Smith HJ, Walters Haygood CL, Arend RC, Leath CA 3rd, Straughn JM Jr. PARP inhibitor maintenance therapy for patients with platinum-sensitive recurrent ovarian cancer: a cost-effectiveness analysis. Gynecol Oncol. 2015;139(1):59–62. https://doi.org/10.1016/j.ygyno.2015.08.013.
Roche. EU approves Roche’s Avastin plus chemotherapy for women with advanced cervical cancer. https://www.roche.com/investors/updates/inv-update-2015-04-08.htm. Accessed 20 Apr 2019.
Chugai Pharmceutical Co. Ltd. Anti-cancer agent “Avastin®,” obtained approval for additional indication of advanced or recurrent cervical cancer. https://www.roche.com/dam/jcr:c7af40b3-c63e-45c1-a38e-b356693712e1/en/inv-update-2016-05-24.pdf. Accessed 20 Apr 2019.
Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6(1):8. https://doi.org/10.1186/s40425-018-0316-z.
Cancer Genome Atlas Research Network, Albert Einstein College of Medicine, Analytical Biological Services, Barretos Cancer Hospital, Baylor College of Medicine, Beckman Research Institute of City of Hope, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378–84. https://doi.org/10.1038/nature21386.
Chung HC, Schellens JHM, Delord J, Perets R, Italiano A, Shapira-Frommer R, et al. Pembrolizumab treatment of advanced cervical cancer: updated results from the phase 2 KEYNOTE-158 study. J Clin Oncol. 2018;36(15_Suppl.):5522. https://doi.org/10.1200/jco.2018.36.15_suppl.5522.
Liu C, Lu J, Tian H, Du W, Zhao L, Feng J, et al. Increased expression of PDL1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol Med Rep. 2017;15(3):1063–70. https://doi.org/10.3892/mmr.2017.6102.
Yamashita H, Nakayama K, Ishikawa M, Nakamura K, Ishibashi T, Sanuki K, et al. Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget. 2018;9(5):5652–64. https://doi.org/10.18632/oncotarget.23790.
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–8.
Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6(1):35. https://doi.org/10.1186/s40425-018-0342-x.
Merck Newsroom. Merck’s Keytruda® (pembrolizumab) receives five new approvals in Japan, including in advanced non-small cell lung cancer (NSCLC), as adjuvant therapy for melanoma, and in advanced microsatellite instability-high (MSI-H) tumors. https://www.mrknewsroom.com/news-release/oncology/mercks-keytruda-pembrolizumab-receives-five-new-approvals-japan-including-adva. Accessed 20 Apr 2019.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. https://doi.org/10.1056/NEJMoa1500596.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13. https://doi.org/10.1126/science.aan6733.
Diaz LA Jr, Marabelle A, Kim TW, Geva R, Van Cutsem E, André T, et al. Efficacy of pembrolizumab in phase 2 KEYNOTE-164 and KEYNOTE-158 studies of microsatellite instability high cancers. Ann Oncol. 2017;28(5_Suppl.):386P.
Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol. 2017;35(22):2535–41. https://doi.org/10.1200/jco.2017.72.5952.
Mittica G, Ghisoni E, Giannone G, Aglietta M, Genta S, Valabrega G. Checkpoint inhibitors in endometrial cancer: preclinical rationale and clinical activity. Oncotarget. 2017;8(52):90532–44. https://doi.org/10.18632/oncotarget.20042.
National Comprehensive Cancer Network. NCCN guidelines version 3. 2019. Endometrial carcinoma. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed Apr 2019.
Chapel DB, Yamada SD, Cowan M, Lastra RR. Immunohistochemistry for mismatch repair protein deficiency in endometrioid endometrial carcinoma yields equivalent results when performed on endometrial biopsy/curettage or hysterectomy specimens. Gynecol Oncol. 2018;149(3):570–4. https://doi.org/10.1016/j.ygyno.2018.04.005.
ClinicalTrials.gov. A study of niraparib maintenance treatment in patients with advanced ovarian cancer following response on front-line platinum-based chemotherapy. 2018. https://clinicaltrials.gov/ct2/show/NCT02655016?term=PRIMA&cond=ovarian&draw=1&rank=4. Accessed 8 Nov 2018.
Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–22. https://doi.org/10.1200/JCO.2015.62.3397.
ClinicalTrials.gov. A study in ovarian cancer patients evaluating rucaparib and nivolumab as maintenance treatment following response to front-line platinum-based chemotherapy (ATHENA). 2018. Available from:https://clinicaltrials.gov/ct2/show/NCT03522246?term=NCT03522246&rank=1. Accessed 8 Nov 2018.
Konstantinopoulos PA, Waggoner SE, Vidal GA, Mita MM, Fleming GF, Holloway RW, et al. TOPACIO/Keynote-162 (NCT02657889): a phase 1/2 study of niraparib + pembrolizumab in patients (pts) with advanced triple-negative breast cancer or recurrent ovarian cancer (ROC): results from ROC cohort. J Clin Oncol. 2018;36(15_Suppl):106. https://doi.org/10.1200/jco.2018.36.15_suppl.106.
De Witt Hamer PC, Mir SE, Noske D, Van Noorden CJ, Wurdinger T. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011;17(13):4200–7. https://doi.org/10.1158/1078-0432.CCR-10-2537.
Do K, Wilsker D, Ji J, Zlott J, Freshwater T, Kinders RJ, et al. Phase I study of single-agent AZD1775 (MK-1775), a Wee1 kinase inhibitor, in patients with refractory solid tumors. J Clin Oncol. 2015;33(30):3409–15. https://doi.org/10.1200/JCO.2014.60.4009.
Leijen S, van Geel RM, Sonke GS, de Jong D, Rosenberg EH, Marchetti S, et al. Phase II study of WEE1 inhibitor AZD1775 plus carboplatin in patients with TP53-mutated ovarian cancer refractory or resistant to first-line therapy within 3 months. J Clin Oncol. 2016;34(36):4354–61. https://doi.org/10.1200/JCO.2016.67.5942.
ClinicalTrials.gov. Adavosertib with or without olaparib in treating participants with recurrent ovarian, primary peritoneal, or fallopian tube cancer. 2018. Available from:https://clinicaltrials.gov/ct2/show/NCT03579316?term=NCT03579316&rank=1. Accessed 15 Nov 2018.
Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15(11):1207–14. https://doi.org/10.1016/S1470-2045(14)70391-2.
ClinicalTrials.gov. Olaparib ± cediranib or chemotherapy in patients with BRCA mutated platinum-resistant ovarian cancer (OCTOVA). 2018. Available from:https://clinicaltrials.gov/ct2/show/NCT03117933?term=NCT03117933&rank=1. Accessed 15 Nov 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of interest
Kathy Pan, Jun Gong, and Karen Huynh have no conflicts of interest that are directly relevant to the contents of this manuscript. Mihaela Cristea has received a speaker honorarium from AstraZeneca within the past 36 months that is less than US$10,000.
Rights and permissions
About this article
Cite this article
Pan, K., Gong, J., Huynh, K. et al. Current Systemic Treatment Landscape of Advanced Gynecologic Malignancies. Targ Oncol 14, 269–283 (2019). https://doi.org/10.1007/s11523-019-00641-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-019-00641-9