Abstract
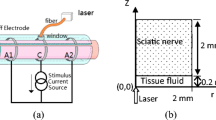
Damaged nerve function can be repaired by applying external stimuli, and the selective stimulation of nerve fibers is the highest goal of nerve functional repair. This paper proposes a method of using multichannel near-infrared lasers to achieve the selective stimulation of axons in different areas in a mixed nerve bundle. An exposed bullfrog sciatic nerve was considered the object of study to realize the selective stimulation. A model was established by using COMSOL Multiphysics to simulate the temperature distribution of nerves under multichannel near-infrared laser stimulation. The results of this model showed that by changing the distance between the laser fiber and the nerve (d) or the power of the 4 lasers (P), the axons at different parts of the nerve bundle may be selectively stimulated. If only the axons located in the center are selected to be activated, it is necessary to set the d and P value in the four directions to the same value. If only axons on the nerve edge are selected for activation, we can reduce the d value of the nearest laser (or increase P) and increase the d value of lasers in other directions (or decrease P). If only axons in the shallow area below the surface between the two lasers are selected for activation, it is necessary to reduce the d value of the laser in two directions close there (or increase P) and increase the d value of the laser in the other two directions (or decrease P). If only the axons of the superficial region on the coordinate axis are activated, the d value of the laser in the farthest direction can be increased (or decrease P) and the d value of the other three lasers can be reduced (or increase P). Moreover, the results of animal experiments further verify the feasibility of our method to realize selective activation of the axons.
Graphical abstract












Similar content being viewed by others
References
Gudnason G, Bruun E, Haugland M (2000) A chip for an implantable neural stimulator. Analog Integr Circ S 22(1):81–89
Richter CP, Matic AI, Well JD, Jansen ED, Walsh JT (2011) Neural stimulation with optical radiation. Laser Photonics Rev. 5(1):68–80
Wells J, Kao C, Jansen ED, Konrad P, Mahadevan-Jansen A (2014) Application of infrared light for in vivo neural stimulation. J Biomed Opt. 10(6):064003
Qiang S, Xueliang L, Dazong J, Changfeng T (2001) A bipolar method for selective activation of nerve fibers using biphasic rectangular pulses. Chin J Biomed Eng 20(6):533–540
Fang ZP, Mortimer JT (1991) Selective activations of small motor axons by quasitrapezoidal current pulses. IEEE T Bio-med Eng 38(2):168–174
Freeman DK, Eddington DK, Rizzo J, Fried S (2010) Selective activation of neuronal targets with sinusoidal electric stimulation. J Neurophysiol 104(5):2778–2791
Grill W, Norman S, Bellamkonda R (2009) Implanted neural interfaces: biochallenges and engineered solutions. Biomed Eng 11(1)
Veraart C, Grill W, Mortimer J (1993) Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE T Bio-med Eng 40(7):640–653
Zariffa J, Nagai M, Daskalakis Z, Popovic M (2009) Influence of the number and location of recording contacts on the selectivity of a nerve cuff electrode. IEEE T Reh Eng 17(5):420–427
Butson C, Miller I, Normann R, Clark G (2011) Selective neural activation in a histologically derived model of peripheral nerve. J Neural Eng. 8(3):036009
Wells J, Konrad P, Kao C, Jansen E, Mahadevan-Jansen A (2007) Pulsed laser versus electrical energy for peripheral nerve stimulation. J Neurosci Meth 163(2):326–337
Wells J, Kao C, Konrad P, Milner T, Kim J, Mahadevan-Jansen A et al (2007) Biophysical mechanisms of transientoptical stimulation of peripheral nerve. Biophys J 93(7):2567–2580
Cayce J, Kao C, Malphrus J, Konrad P, Mahadevan-Jansen A, Jansen E (2010) Infrared neural stimulation of thalamocortical brain slices. IEEE J Sel Top Quant 16(3):565–572
Cayce J, Friedman R, Jansen E, Mahavaden-Jansen A, Roe A (2011) Pulsed infrared light alters neural activity in rat somatosensory cortex in vivo. Neuroimage 57(1):155–166
Teudt I, Nevel A, Izzo A, Walsh J, Richter C, Richter C (2007) Optical stimulation of the facial nerve- a new monitoring technique. Laryngoscope 117(9):1641–1647
Izzo A, Walsh J, Ralph H, Webb J, Bendett M, Wells J et al (2008) Laser stimulation of auditory neurons: effect of shorter pulse duration and penetration depth. Biophys J 94(8):3159–3166
Izzo A, Walsh J, Jansen E, Bendett M, Webb J, Ralph H et al (2007) Optical parameter variability in laser nerve stimulation: a study of pulse duration, repetition rate, and wavelength. IEEE Trans Bio-med Eng 54(6):1108–1114
Richter C, Bayon R, Izzo A, Otting M, Suh E, Goyal S et al (2008) Optical stimulation of auditory neurons: effects of acute and chronic deafening. Hearing Res 242(1–2):42–51
Rajguru SM, Rabbitt RR, Matic AI, Highstein SM, Richter CP (2010) Inhibitory and excitatory vestibular afferent responses induced by infrared light stimulation of hair cells, 33rd Midwinter Meeting. Association for Research in Otolaryngology, Anaheim, CA
Chung H, Chung E (2019) Optical stimulation and pacing of the embryonic chicken heart via thulium laser irradiation. Curr Opt Photon 3(1):1–7
Shapiro MG, Homma K, Villarreal S, Richter CP, Bezanilla F (2012) Infrared light excites cells by changing their electrical capacitance. Nat Commun 3:736
Plaksin M, Kimmel E, Shoham S (2017) Thermal transients excite neurons through universal intramembrane mechano-electrical effects. bioRxiv 111724
Rachael TR, Michael RI, Alexander CT, Andrew KW, James BF (2020) Optical stimulation of neural tissue. Healthc Technol Lett 7(3):58–65
Barrett JN, Rincon S, Singh J, Matthewman C, Pasos J, Barrett EF et al (2018) Pulsed infrared releases Ca2þ from the endoplasmic reticulum of cultured spiral ganglion. Neurons 120:509–524
Eom K, Kim J, Choi J, Kang T, Chang J, Byun K et al (2014) Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods. Small 10(19):3853–3857
Wang Y, Guo L (2016) Nanomaterial-enabled neural stimulation. Front Neurosci 10:69
Ren Y, Qi H, Chen Q, Ruan L (2017) Thermal dosage investigation for optimal temperature distribution in gold nanoparticle enhanced photothermal therapy. Int J Heat Mass Tran 106:212–221
Yan C, Yanxiong N, Fang T, Huichai Y, Chu Z, Nan J et al (2007) Numerical simulation of the optical-thermal interaction in vivo layered skin-tissue by Laser. Appl Laser 27(5):382–386
Rickard Vitkaya AY, Seker SS (2016) Study of temperature distribution in light-tissue interaction using the FEM. Turk J Electr Eng Co 24:807–819
Mengxian Y, Zongxia M (2017) Model study of combined electrical and near-infrared neural stimulation on the bullfrog sciatic nerve. Lasers Medl Sci 32(5):1163–1172
Yucel A, Ersen A, Sahin M, Al-Smadi Y (2013) FEA modeling of temperature elevation in neural tissue illuminated by a laser: Transient effects. 6th Annual International IEEE/EMBS Conference on Neural Engineering, pp 1112–1114
Urzova J, Jelinek M (2018) Heat transfer modelling of pulsed laser-tissue interaction. Laser Phys 28(3):036001
Li X (2014) Numerical analysis and experimental research on laser induced thermal effect in bio-tissues. Tianjin University Press, Tianjin, pp 32–40
Mobley J, Vo-Dinh T (2003) Optical properties of tissue. In: Vo-Dinh T (ed) Biomedical photonics handbook. CRC Press, Boca Raton, pp 2-1-2–70
You MX, Mou ZX (2019) Selective stimulation of bullfrog sciatic nerve by gold nanorod assisted combined electrical and near-infrared stimulation. Biomed Microdevice 21:76
Castoro M, Yoo P, Hincapie J, Hamann J, Ruble S, Wolf P et al (2010) Excitation properties of the right cervical vagus nerve in adult dogs. Exp Neurol 227(1):62–68
Stewart J (2003) Peripheral nerve fascicles: anatomy and clinical relevance. Muscle Nerve 28(5):525–541
Funding
This work was supported by the Nature Science Foundation of China Grant (No. 31500796).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
According to Beer-Lambert Law,
Where,
- T:
-
the transmittance (the ratio of transmitted light intensity to incident light intensity).
- K :
-
the extinction coefficient of the solution (L/g/cm).
- C :
-
the concentration of the solution (g/L).
- l :
-
the optical path of the solution (cm).
In this paper, μα = 1/lα.
- μα:
-
the absorption coefficient (1/cm).
- l α :
-
the absorption length (cm).
- l α :
-
is also the optical path when T = 1/e, where AUα = lg(1/T) = lge = 0.43429448. According to Formula (8), AUα = KClα, then
From the measurement results we can see that AU is about 2 at λ = 808 nm, where C = 3e-5 g/l, and l = 1 cm. So according to Formula (8),
Where,
K and C is equal to those in Formula (9) and l is the equal the thickness of the cuvette (l = 1 cm). Then solve formulas (9) and (10), μα = 4.6 (1/cm) = 460 (1/m).
Rights and permissions
About this article
Cite this article
Zhou, R., Mou, Z., Yang, D. et al. Theoretical simulation of the selective stimulation of axons in different areas of a nerve bundle by multichannel near-infrared lasers. Med Biol Eng Comput 60, 205–220 (2022). https://doi.org/10.1007/s11517-021-02475-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02475-y