Abstract
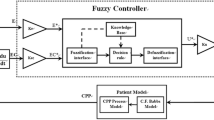
Chest compression (CC) is a significant emergency medical procedure for maintaining circulation during cardiac arrest. Although CC produces the necessary blood flow for patients with heart arrest, improperly deep CC will contribute significantly to the risk of chest injury. In this paper, an optimal CC closed-loop controller for a mechanical chest compressor (OCC-MCC) was developed to provide an effective trade-off between the benefit of improved blood perfusion and the risk of ribs fracture. The trade-off performance of the OCC-MCC during real automatic mechanical CCs was evaluated by comparing the OCC-MCC and the traditional mechanical CC method (TMCM) with a human circulation hardware model based on hardware simulations. A benefit factor (BF), risk factor (RF) and benefit versus risk index (BRI) were introduced in this paper for the comprehensive evaluation of risk and benefit. The OCC-MCC was developed using the LabVIEW control platform and the mechanical chest compressor (MCC) controller. PID control is also employed by MCC for effective compression depth regulation. In addition, the physiological parameters model for MCC was built based on a digital signal processor for hardware simulations. A comparison between the OCC-MCC and TMCM was then performed based on the simulation test platform which is composed of the MCC, LabVIEW control platform, physiological parameters model for MCC and the manikin. Compared with the TMCM, the OCC-MCC obtained a better trade-off and a higher BRI in seven out of a total of nine cases. With a higher mean value of cardiac output (1.35 L/min) and partial pressure of end-tidal CO2 (15.7 mmHg), the OCC-MCC obtained a larger blood flow and higher BF than TMCM (5.19 vs. 3.41) in six out of a total of nine cases. Although it is relatively difficult to maintain a stable CC depth when the chest is stiff, the OCC-MCC is still superior to the TMCM for performing safe and effective CC during CPR. The OCC-MCC is superior to the TMCM in performing safe and effective CC during CPR and can be incorporated into the current version of mechanical CC devices for high quality CPR, in both in-hospital and out-of-hospital CPR settings.






Similar content being viewed by others
References
Abu-Rmileh A, Garcia-Gabin W, Zambrano D et al (2010) A robust sliding mode controller with internal model for closed-loop artificial pancreas. Med Biol Eng Comput 48:1191–1201
Babbs CF (1999) CPR techniques that combine chest and abdominal compression and decompression. Circulation 100:2146–2152
Babbs CF (2005) Effects of an impedance threshold valve upon hemodynamic in Standard CPR: studies in a refined computational model. Resuscitation 66:335–345
Babbs CF (2006) Design of near-optimal waveforms for chest and abdominal compression and decompression in CPR using computer simulated evolution. Resuscitation 68:277–293
Babbs CF, Kemeny AE, Quan W, Freeman G (2008) A new paradigm for human resuscitation research using intelligent devices. Resuscitation 77:306–315
Black CJ, Busuttil A, Roberson C (2004) Chest wall injuries following cardiopulmonary resuscitation. Resuscitation 63:339–343
Callaham M, Barton C (1990) Prediction of outcome of cardiopulmonary resuscitation from end-tidal carbon dioxide concentration. Crit Care Med 18:258–362
Duma SM, Kemper AR, Stitzel JD, Mcnally C, Kennedy EA, Matsuoka F (2011) Rib fracture timing in dynamic belt tests with human cadavers. Clin Anat 24:327–338
Edelson DP, Abella BS, Johansen JK, Wik L, Myklebust H, Barry AM, Merchant RM, Hoek TL, Steen PA, Becker LB (2006) Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation 71:137–145
Edelson DP, Litzinger B, Arora V, Walsh D, Kim S, Lauderdale DS, Vanden Hoek TL, Becker LB, Abella BS (2008) Improving in-hospital cardiac arrest process and outcomes with performance debriefing. Arch Intern Med 168:1063–1069
Fitzgerald KR, Babbs CF, Frissora HA et al (1981) Cardiac output during cardiopulmonary resuscitation at various compression rates and durations. Am J Physiol 241:H442–H448
Grmec S, Klemen P (2001) Does the end-tidal carbon dioxide (EtCO2) concentration have prognostic value during out-of-hospital cardiac arrest? Eur J Emerg. Med 8:263–269
Hoke RS, Chamberlain D (2004) Skeletal chest injuries secondary to cardiopulmonary resuscitation. Resuscitation 63:327–338
Johansen JK, Myklebust H, Wik L, Fellows B, Svensson L, Sorebo H, Steen PA (2006) Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: a prospective interventional study. Resuscitation 71:283–292
Kemper AR, Kennedy EA, Mcnally C, Manoogian SJ, Stitzel JD, Duma SM (2011) Reducing chest injuries in automobile collisions: rib fracture timing and implications for thoracic injury criteria. Ann Biomed Eng 39:2141–2151
Lewis LM, Stothert J, Standeven J, Chandel B, Kurtz M, Fortney J (1992) Correlation of end-tidal CO2 to cerebral perfusion during CPR. Ann Emerg Med 21:1131–1134
Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, Passman RS, White RD, Hess EP, Tang W, Davis D, Sinz E, Morrison LJ (2010) Adult advanced cardiovascular life support. 2010 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular Care. Circulation 122:S729–S767
Odegaard S, Kramer-Johansen J, Bromley A et al (2007) Chest compressions by ambulance personnel on chests with variable stiffness: abilities and attitudes. Resuscitation 74:127–134
Paradis NA, Martin GB, Goetting MG et al (1989) Simultaneous aortic, jugular bulb, and right atrial pressures during cardiopulmonary resuscitation in humans. Circulation 80:361–368
Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, Nowak RM (1990) Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 263:1106–1113
Rivers EP, Martin GB, Rady MY, Smithline HA, Appleton TJ, Nowak RM (1992) Coronary perfusion pressure, end-tidal carbon dioxide concentration and continuous central venous oxygen saturation monitoring as a predictor of outcome during human CPR. Crit Care Med 20:S85–S86
Sanders A, Atlas M, Ewy G, Kern K, Bragg S (1985) Expired pCO2 as an index of coronary perfusion pressure. Am J Emerg Med 3:147–149
Sanders AB, Ewy GA, Bragg S, Atlas M, Kern KB (1985) Expired PCO2 as a prognostic indicator of successful resuscitation from cardiac arrest. Ann Emerg Med 14:948–952
Sanders AB, Kern KB, Otto CW, Milander MM, Ewy GA (1989) End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA 262:1347–1351
Tsitlik JE, Weisfeldt ML, Chandra N, Effron MB, Halperin HR, Levin HR (1983) Elastic properties of the human chest during cardiopulmonary resuscitation. Crit Care Med 11:685–692
Varon J, Marik PE, Fromm RE (1998) Cardiopulmonary resuscitation: a review for clinicians. Resuscitation 36:133–145
Werner JA, Greene L, Janko CL, Cobb LA (1981) Visualization of cardiac valve motion in man during external chest compression using twodimensional echocardiography. Circulation 63:1417–1421
Zhang G, Zheng J, Wu J, Wu T (2012) An optimal closed-loop control strategy for mechanical chest compression devices: a trade-off between the risk of chest injury and the benefit of enhanced blood flow. Comput Methods Program Biomed 108:288–298
Zhang G, Zheng J, Wu J, Wu T (2012) Closed-loop controller for chest compression during cardiopulmonary resuscitation: a computer simulation study. J Med Biol Eng 32:313–322
Conflict of interest
None of the authors has a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: The benefit factor and risk factor for CC
1.1 Benefit factor
To quantify the benefit of blood flow, it is reasonable to use the PETCO2 as a benefit factor, which is quantified from 0 to 10. The BF is divided into three levels according to the benefit for ROSC represented by the PETCO2 (Fig. 1).
Level 1, where the PETCO2 value ranges from 0 to 10 mmHg, is the lowest level. Clinical studies have revealed that all patients who were successfully resuscitated had a minimum PETCO2 value of 10 mmHg, and no patient with a PETCO2 value of less than 10 mmHg was resuscitated [12, 24]. CC resulting in a PETCO2 value in Level 1 does not provide any benefit for ROSC. Therefore, the BF value for Level 1 is zero.
Level 2, with a PETCO2 value ranging from 10 to 15 mmHg, is the medium level. Clinical studies have found several different average PETCO2 values ensuring the resuscitation of patients, including 18.8 [12], 15 [24] and 13 mmHg [21]. In this study, 15 mmHg was regarded as the average PETCO2 value for predicting a patient’s ROSC. Because the PETCO2 value from 10 to 15 mmHg is between the minimal and average PETCO2 ROSC value, CC causing a PETCO2 value in Level 2 benefits ROSC moderately.
Level 3, with a PETCO2 value from 15 to 21 mmHg or more than 21 mmHg, is the highest level. Human studies have found that the maximal PETCO2 value predicting patients’ ROSC is from 16 to 27 mmHg [12, 21, 24]. The median of these maximal PETCO2, 21 mmHg, is regarded as the value representing the top of the BF, and CC producing a PETCO2 value of more than 21 mmHg is also believed to have the top BF value, 10.
With the basic concepts described above, a BF value can be calculated as follows:
In (3), the max(A, B) function is used to find the maximum value between A and B; the min(A, B) function is used to find the minimum value between A and B.
1.2 Risk factor
It is possible that older individuals have stiffer chests and greater risk of chest injuries during CPR [6, 13]. The stiffer chest is more likely to be injured during CPR. Therefore, it is reasonable to predict the risk of skeletal chest injuries using a chest stiffness value (K chest) during CPR. In this study, to quantify the risk of chest injuries, a risk factor was introduced and quantized from 0 to 10 according the chest stiffness value.
So far, few studies have investigated the quantitative relationship between K chest and the degree of skeletal chest injury risk. It is impossible to use K chest values achieved from clinical studies to divide the RF, as was mentioned in “Benefit factor” section. However, it is reasonable to use several key K typical values, which reflect averages of human K chest values with different compression depth, as the boundaries of the RF [25]. These key K typical values can be calculated using the means of β and γ in Eq. (9) with the combination of 2010 AHA guidelines and CC experiences, and they are displayed below:
here, K5, K5.5, K6 and K6.5 are K typical values with a CC depth of 5, 5.5, 6 and 6.5, respectively.
The classification of RF, which is carried out according Eq. (4), is shown in Fig. 2.
To obtain a high quality of CC, the 2010 AHA guidelines state that the adult sternum should be depressed at least 5 cm. According to this requirement, it is believed that the risk presented by a compression with a depth of less than 5 cm is minimal for a typical human chest. In clinical practice, the manual CC depth is rarely greater than 6 cm. CC with a depth greater than 6 cm, which is excessively deep for most patients, is likely to break patients’ sterna and ribs. Therefore, Level 1 with a K chest from K0 to K5 is the lowest in terms of risk. The RF value in Level 1 is zero. Level 4, with a K chest from K6 to K6.5 or more than K6.5, is the highest level of the RF. CC with a K chest between K5 and K6 is believed to present a moderate risk of chest injury for the patient. To achieve a better outcome for the OCCCS, moderate risk was divided into small risk and large risk, with the boundary at K5.5. Level 2, with a K chest from K5 to K5.5, represents a small risk, while Level 3, with a K chest from K5.5 to K6, represents a large risk.
With the basic concepts described above, an RF value can be calculated as follows:
The RF proposed in this study is a provisional one, which will be refined in view of future data relating chest stiffness to risk in real patients.
Appendix 2: Optimal closed-loop control algorithm
To achieve dramatically effective CC, an optimal closed-loop control algorithm was developed based on the following five design rules:
-
CC is optimized by regulating the compression depth at the standard compression rate of 100/min recommended by the 2010 AHA guidelines.
-
Both the benefit and the risk should be given full consideration using the feedback of the PETCO2 and K chest.
-
An extremely dangerous situation should be avoided by OCCCS design practices. A PETCO2 less than 10 mmHg (PETCO2 in Level 1) and a K chest value higher than K6 (K chest in Level 4) should be avoided if possible.
-
The PETCO2 should be increased as much as possible without serious chest injuries (K chest in Level 4).
-
In accordance with the 2010 AHA guidelines, the initial compression depth is set to 5 cm. The final optimal compression depth is the sum of 5 cm and the optimal CC depth offset achieved using OCCCS.
The algorithm for an OCCCS based on fuzzy control strategy, which is obtained by summarizing the design rules presented above and a novel closed-loop control method established by Amjad, can be described by the following 12 rules [1].
- #1:
-
IF Benefit is in Level 1 and Risk is in Level 1, THEN Offset is PB
- #2:
-
IF Benefit is in Level 1 and Risk is in Level 2, THEN Offset is PM
- #3:
-
IF Benefit is in Level 1 and Risk is in Level 3, THEN Offset is PS
- #4:
-
IF Benefit is in Level 1 and Risk is in Level 4, THEN Offset is ZO
- #5:
-
IF Benefit is in Level 2 and Risk is in Level 1, THEN Offset is PM
- #6:
-
IF Benefit is in Level 2 and Risk is in Level 2, THEN Offset is PS
- #7:
-
IF Benefit is in Level 2 and Risk is in Level 3, THEN Offset is ZO
- #8:
-
IF Benefit is in Level 2 and Risk is in Level 4, THEN Offset is NS
- #9:
-
IF Benefit is in Level 3 and Risk is in Level 1, THEN Offset is PS
- #10:
-
IF Benefit is in Level 3 and Risk is in Level 2, THEN Offset is ZO
- #11:
-
IF Benefit is in Level 3 and Risk is in Level 3, THEN Offset is NS
- #12:
-
IF Benefit is in Level 3 and Risk is in Level 4, THEN Offset is NM
where Offset is short for the CC depth offset. As a fuzzy controller output, offset has seven fuzzy items: negative big (NB), negative medium (NM), negative small (NS), approximately zero (ZO), positive small (PS), positive medium (PM) and positive big (PB). The CC depth offset surfaces as a function of the PETCO2 and chest stiffness for an OCCCS is presented by Fig. 7.
Appendix 3: PETCO2 and chest stiffness simulation
3.1 PETCO2 simulation
In clinical studies, PETCO2 appears correlated with coronary perfusion pressure (CPP). To investigate the relationship between CPP and PETCO2, Arthur B. Sanders carried out a series of CPR studies with dogs. The results of these studies indicated that PETOC2 is positively correlated with CPP in the canine model of CPR (r = 0.91, P < 0.01). The relationship between PETCO2 and CPP can be approximately represented by a linear regression equation [22, 23]. Based on the results of studies performed by Sanders, the relationship between PETCO2 and CPP in the human model of CPR may be approximately described by a linear equation as follows:
To explore the relationship between CPP, PETCO2 and ROSC in the studies implemented by Norman A. Paradis and Arthur B. Sanders, cardiac arrests in numerous patients were monitored during CPR [20, 24]. The results are shown in Table 4.
The values of the variables α and β in Eq. (6) can be calculated with a system of linear equations using the data from Table 4. Thus, the mathematical function of the relationship between CPP and PETCO2, which will be referred to as the CPP-PETCO2 model, is given by the expression
The validation results indicate that the data from the CPP-PETCO2 model with a PETCO2 absolute mean error of 1 mmHg and a CPP absolute mean error of 2 mmHg closely approximate the clinical results in terms of CPP and PETCO2 [28].
Therefore, the PETCO2 resulting from different CC parameters can be simulated by the combination of CPP from Babbs’ model [2–4] and the CPP-PETCO2 model.
3.2 Chest stiffness simulation
To measure the actual elasticity properties of the adult human chest during cyclical compression, Joshua E. Tsitlik implemented clinical studies including ten patients and measured compression force and depth during CPR [25]. The sternal elastic characteristic can be satisfactorily described with a second degree polynomial
where F and x are the CC force and CC depth, respectively, β = 54.9 ± 29.4 (mean ± SD) N/cm is the initial elasticity and γ = 10.8 ± 4.1 (mean ± SD) N/cm2 is the posterior resiliency.
To characterize the ability of the human chest to resist a compression force, chest stiffness (K chest) is defined. K chest, which is the resistance of human chest to a unit of deformation by CC force, is a dynamic variable depending on the CC depth and defined as the derivative of the elastic force F (CC force in Eq. (8)) with respect to displacement x (CC depth in Eq. (8)), as follows:
The definitions of F, x, β and γ in Eq. (9) are the same as those in Eq. (9). Chest stiffness can be simulated using the Eq. (9) with CC depth.
Rights and permissions
About this article
Cite this article
Zhang, G., Wu, T., Song, Z. et al. A mechanical chest compressor closed-loop controller with an effective trade-off between blood flow improvement and ribs fracture reduction. Med Biol Eng Comput 53, 487–497 (2015). https://doi.org/10.1007/s11517-015-1258-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-015-1258-y