Abstract
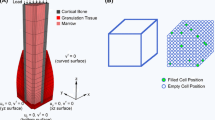
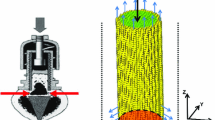
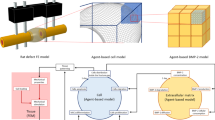
We have developed a mathematical model that allows simulation of oxygen distribution in a bone defect as a tool to explore the likely effects of local changes in cell concentration, defect size or geometry, local oxygen delivery with oxygen-generating biomaterials (OGBs), and changes in the rate of oxygen consumption by cells within a defect. Experimental data for the oxygen release rate from an OGB and the oxygen consumption rate of a transplanted cell population are incorporated into the model. With these data, model simulations allow prediction of spatiotemporal oxygen concentration within a given defect and the sensitivity of oxygen tension to changes in critical variables. This information may help to minimize the number of experiments in animal models that determine the optimal combinations of cells, scaffolds, and OGBs in the design of current and future bone regeneration strategies. Bone marrow-derived nucleated cell data suggest that oxygen consumption is dependent on oxygen concentration. OGB oxygen release is shown to be a time-dependent function that must be measured for accurate simulation. Simulations quantify the dependency of oxygen gradients in an avascular defect on cell concentration, cell oxygen consumption rate, OGB oxygen generation rate, and OGB geometry.






Similar content being viewed by others
References
Abdi SI, Ng SM, Lim JO (2011) An enzyme-modulated oxygen-producing micro-system for regenerative therapeutics. Int J Pharm 409:203–205
Calori GM, Mazza E, Colombo M, Ripamonti C (2011) The use of bone-graft substitutes in large bone defects: any specific needs? Injury 42(Suppl 2):S56–S63
Fayaz HC, Giannoudis PV, Vrahas MS, Smith RM, Moran C, Pape HC, Krettek C, Jupiter JB (2011) The role of stem cells in fracture healing and nonunion. Int Orthop 35:1587–1597
Han P, Bartels DM (1996) Temperature dependence of oxygen diffusion in H2O and D2O. J Phys Chem 100:5597–5602
Harris LD, Kim BS, Mooney DJ (1998) Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res 42:396–402
Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ (2007) Oxygen producing biomaterials for tissue regeneration. Biomaterials 28:4628–4634
Heppenstall RB, Grislis G, Hunt TK (1975) Tissue gas tensions and oxygen consumption in healing bone defects. Clin Orthop Relat Res 106:357–365
Huang CY, Yuan TY, Jackson AR, Hazbun L, Fraker C, Gu WY (2007) Effects of low glucose concentrations on oxygen consumption rates of intervertebral disc cells. Spine 32:2063–2069
Jain HV, Moldovan NI, Byrne HM (2012) Modeling stem/progenitor cell-induced neovascularization and oxygenation around solid implants. Tissue Eng Part C Methods 18:487–495
Jonitz A, Lochner K, Lindner T, Hansmann D, Marrot A, Bader R (2011) Oxygen consumption, acidification and migration capacity of human primary osteoblasts within a three-dimensional tantalum scaffold. J Mater Sci Mater Med 22:2089–2095
Kiaer T, Dahl B, Lausten G (1992) Partial pressures of oxygen and carbon dioxide in bone and their correlation with bone-blood flow: effect of decreased arterial supply and venous congestion on intraosseous oxygen and carbon dioxide in an animal model. J Orthop Res 10:807–812
Malda J, Rouwkema J, Martens DE, Le Comte EP, Kooy FK, Tramper J, van Blitterswijk CA, Riesle J (2004) Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. Biotechnol Bioeng 86:9–18
Muschler GF, Midura RJ (2002) Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res 395:66–80
Muschler GF, Nakamoto C, Griffith LG (2004) Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am 86-A:1541–1558
Muschler GF, Raut VP, Patterson TE, Wenke JC, Hollinger JO (2010) The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng Part B Rev 16:123–145
Nandi SK, Roy S, Mukherjee P, Kundu B, De DK, Basu D (2010) Orthopaedic applications of bone graft and graft substitutes: a review. Indian J Med Res 132:15–30
Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS (2009) Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 30:757–762
Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL (2012) Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci USA 109:4245–4250
Powell KA, Nakamoto C, Villarruel S, Boehm C, Muschler G (2007) Quantitative image analysis of connective tissue progenitors. Anal Quant Cytol Histol 29:112–121
Sato K, Moy OJ, Peimer CA, Nakamura T, Howard C, Ko SH, Lee TC, Nishiwaki Y (2011) An experimental study on costal osteochondral graft. Osteoarthr Cartil 20:172–183
Sheridan MH, Shea LD, Peters MC, Mooney DJ (2000) Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J Control Release 64:91–102
Takigami H, Kumagai K, Latson L, Togawa D, Bauer T, Powell K, Butler RS, Muschler GF (2007) Bone formation following OP-1 implantation is improved by addition of autogenous bone marrow cells in a canine femur defect model. J Orthop Res 25:1333–1342
Villarruel SM, Boehm CA, Pennington M, Bryan JA, Powell KA, Muschler GF (2008) The effect of oxygen tension on the in vitro assay of human osteoblastic connective tissue progenitor cells. J Orthop Res 26:1390–1397
Wang J, Zhu Y, Bawa HK, Ng G, Wu Y, Libera M, van der Mei HC, Busscher HJ, Yu X (2010) Oxygen-generating nanofiber cell scaffolds with antimicrobial properties. ACS Appl Mater Interfaces 3:67–73
Wang L, Wilshaw SP, Korossis S, Fisher J, Jin Z, Ingham E (2009) Factors influencing the oxygen consumption rate of aortic valve interstitial cells: application to tissue engineering. Tissue Eng Part C Methods 15:355–363
Wehrhan F, Amann K, Molenberg A, Lutz R, Neukam FW, Schlegel KA (2011) PEG matrix enables cell-mediated local BMP-2 gene delivery and increased bone formation in a porcine critical size defect model of craniofacial bone regeneration. Clin Oral Implants Res 23:805–813
Wilson SM, Goldwasser MS, Clark SG, Monaco E, Bionaz M, Hurley WL, Rodriguez-Zas S, Feng L, Dymon Z, Wheeler MB (2012) Adipose-derived mesenchymal stem cells enhance healing of mandibular defects in the ramus of swine. J Oral Maxillofac Surg 70:e193–e203
Zhao F, Pathi P, Grayson W, Xing Q, Locke BR, Ma T (2005) Effects of oxygen transport on 3-d human mesenchymal stem cell metabolic activity in perfusion and static cultures: experiments and mathematical model. Biotechnol Prog 21:1269–1280
Acknowledgments
This work was supported by National Institutes of Health T32 AR050959, the Cleveland Clinic Foundation, and the Armed Forces Institute of Regenerative Medicine (AFIRM). AFIRM is managed and funded through the US Army Medical Research and Materiel Command (MRMC). The AFIRM has additional funding from the US Navy, the Office of Naval Research, the US Air Force Office of the Surgeon General, the National Institutes of Health, the Veterans Administration, the Cleveland Clinic, and local public and private matching funds. The AFIRM contribution to this publication was supported by a subcontract from Rutgers University, Department of Chemistry and Chemical Biology/NJ Center for Biomaterials, under Cooperative Agreement No. WSIXWH-08-2-0034 from the US Department of Defense, US Army Medical Research Acquisition.
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heylman, C.M., Santoso, S., Krebs, M.D. et al. Modeling and experimental methods to predict oxygen distribution in bone defects following cell transplantation. Med Biol Eng Comput 52, 321–330 (2014). https://doi.org/10.1007/s11517-013-1133-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-013-1133-7