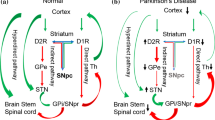
Abstract
Development of new therapeutic targets for neurodegenerative disorders has been hampered by a reliance on post mortem tissue that is representative of end-stage disease, or on animal models that fail to provide faithful analogs. However, rapid advances in cellular genetic reprogramming, in particular the induction of somatic cells into stem cells, or directly into neurons, has led to intense interest in modeling of human neurodegeneration in vitro. Here, we critically review current methods and recent progress in cellular models of Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis. Several challenges are identified, including technical variability, lack of degenerative phenotypes, neurodevelopmental age and establishing ground truths for models of sporadic disease. Recommendations for evaluating neurodegenerative cellular models are proposed along with suggestions for future research.
Similar content being viewed by others
References
Anokye-Danso F, Snitow M, Morrisey E E (2012). How microRNAs facilitate reprogramming to pluripotency. J Cell Sci, 125(Pt 18): 4179–4187
Boulting G L, Kiskinis E, Croft G F, Amoroso M W, Oakley D H, Wainger B J, Williams D J, Kahler D J, Yamaki M, Davidow L, Rodolfa C T, Dimos J T, Mikkilineni S, MacDermott A B, Woolf C J, Henderson C E, Wichterle H, Eggan K (2011). A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol, 29(3): 279–286
Braak H, Braak E 1998. Evolution of neuronal changes in the course of Alzheimer’s disease. In: Jellinger K, Fazekas F, Windisch M (eds.) Ageing and Dementia. Vienna: Springer Vienna
Braak H, Brettschneider J, Ludolph A C, Lee VM, Trojanowski J Q, Del Tredici K (2013). Amyotrophic lateral sclerosis—a model of corticofugal axonal spread. Nat Rev Neurol, 9(12): 708–714
Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K (2004). Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res, 318(1): 121–134
Brayne C (2007). The elephant in the room- healthy brains in later life, epidemiology and public health. Nat Rev Neurosci, 8(3): 233–239
Breitner J C (2015). Comment: Yet another “disconnect” between amyloid and Alzheimer disease? Neurology, 85 (8): 698
Bruijn L I, Becher M W, Lee M K, Anderson K L, Jenkins N A, Copeland N G, Sisodia S S, Rothstein J D, Borchelt D R, Price D L, Cleveland D W (1997). ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron, 18(2): 327–338
Burkhardt M F, Martinez F J, Wright S, Ramos C, Volfson D, Mason M, Garnes J, Dang V, Lievers J, Shoukat-Mumtaz U, Martinez R, Gai H, Blake R, Vaisberg E, Grskovic M, Johnson C, Irion S, Bright J, Cooper B, Nguyen L, Griswold-Prenner I, Javaherian A (2013). A cellular model for sporadic ALS using patient-derived induced pluripotent stem cells. Mol Cell Neurosci, 56: 355–364
Byers B, Cord B, Nguyen H N, Schüle B, Fenno L, Lee P C, Deisseroth K, Langston J W, Pera R R, Palmer T D (2011). SNCA triplication Parkinson’s patient’s iPSC-derived DA neurons accumulate a- synuclein and are susceptible to oxidative stress. PLoS ONE, 6 (11): e26159–e26159
Byrne J A (2008). Generation of isogenic pluripotent stem cells. Hum Mol Genet, 17(R1): R37–R41
Cairns N J, Perrin R J, Franklin E E, Carter D, Vincent B, Xie M, Bateman R J, Benzinger T, Friedrichsen K, Brooks W S, Halliday G M, McLean C, Ghetti B, Morris J C, the Alzheimer Disease Neuroimaging Initiative, the Dominantly Inherited Alzheimer Network (2015). Neuropathologic assessment of participants in two multi-center longitudinal observational studies: the Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN). Neuropathology, 35(4): 390–400
Choi S H, Kim Y H, Hebisch M, Sliwinski C, Lee S, D’Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee J B, Zhang C, Wainger B J, Peitz M, Kovacs D M, Woolf C J, Wagner S L, Tanzi R E, Kim D Y (2014). A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature, 515(7526): 274–278
Coan G, Mitchell C S (2015). An assessment of possible neuropathology and clinical relationships in 46 Sporadic amyotrophic lateral sclerosis patient autopsies. Neurodegener Dis, 15(5): 301–312
Collins P Y, Patel V, Joestl S S, March D, Insel T R, Daar A S, Anderson W, Dhansay MA, Phillips A, Shurin S, Walport M, Ewart W, Savill S J, Bordin I A, Costello E J, Durkin M, Fairburn C, Glass R I, Hall W, Huang Y, Hyman S E, Jamison K, Kaaya S, Kapur S, Kleinman A, Ogunniyi A, Otero-Ojeda A, Poo M M, Ravindranath V, Sahakian B J, Saxena S, Singer P A, Stein D J, the Scientific Advisory Board and the Executive Committee of the Grand Challenges on Global Mental Health (2011). Grand challenges in global mental health. Nature, 475 (7354): 27–30
Crystal H A, Dickson D, Sliwinski M, Masur D, Blau A, Lipton R B (1996). Associations of status and change measures of neuropsychological function with pathologic changes in elderly, originally nondemented subjects. Arch Neurol, 53(1): 82–87
Dauer W, Przedborski S (2003). Parkinson’s disease: mechanisms and models. Neuron, 39(6): 889–909
Duan L, Bhattacharyya B J, Belmadani A, Pan L, Miller R J, Kessler J A (2014). Stem cell derived basal forebrain cholinergic neurons from Alzheimer’s disease patients are more susceptible to cell death. Mol Neurodegener, 9(1): 3–3
Fernández-Santiago R, Carballo-Carbajal I, Castellano G, Torrent R, Richaud Y, Sánchez-Danés A, Vilarrasa-Blasi R, Sánchez-Pla A, Mosquera J L, Soriano J, López-Barneo J, Canals J M, Alberch J, Raya Á, Vila M, Consiglio A, Martín-Subero J I, Ezquerra M, Tolosa E (2015). Aberrant epigenome in iPSC-derived dopaminergic neurons from Parkinson’s disease patients. EMBO Mol Med, 7 (12): 1529–1546
Gandhi S, Wood NW (2010). Genome-wide association studies: the key to unlocking neurodegeneration? Nat Neurosci, 13(7): 789–794
Gurney M E, Pu H, Chiu A Y, Dal Canto M C, Polchow C Y, Alexander D D, Caliendo J, Hentati A, Kwon YW, Deng H X, et (1994). Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science, 264(5166): 1772–1775
Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere M L, Pahwa J S, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan A R, Lovestone S, Powell J, Proitsi P, Lupton M K, Brayne C, Rubinsztein D C, Gill M, Lawlor B, Lynch A, Morgan K, Brown K S, Passmore P A, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith A D, Love S, Kehoe P G, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, Heun R, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate A M, Kauwe J S, Cruchaga C, Nowotny P, Morris J C, Mayo K, Sleegers K, Bettens K, Engelborghs S, de Deyn P P, van Broeckhoven C, Livingston G, Bass N J, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw C E, Tsolaki M, Singleton A B, Guerreiro R, Mühleisen T W, Nöthen M M, Moebus S, Jöckel K H, Klopp N, Wichmann H E, Carrasquillo MM, Pankratz V S, Younkin S G, Holmans P A, O’Donovan M, Owen M J, Williams J (2009). Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet, 41(10): 1088–1093
Honda M, Minami I, Tooi N, Morone N, Nishioka H, Uemura K, Kinoshita A, Heuser J E, Nakatsuji N, Aiba K (2016). The modeling of Alzheimer’s disease by the overexpression of mutant Presenilin 1 in human embryonic stem cells. Biochem Biophys Res Commun, 469(3): 587–592
Hossini A M, Megges M, Prigione A, Lichtner B, Toliat M R, Wruck W, Schröter F, Nuernberg P, Kroll H, Makrantonaki E, Zouboulis C C, Adjaye J (2015). Induced pluripotent stem cell-derived neuronal cells from a sporadic Alzheimer’s disease donor as a model for investigating AD-associated gene regulatory networks. BMC Genomics, 16 (1): 84
Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H (2013). Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science, 341(6146): 651–654
Hu W, Qiu B, Guan W, Wang Q, Wang M, Li W, Gao L, Shen L, Huang Y, Xie G, Zhao H, Jin Y, Tang B, Yu Y, Zhao J, Pei G (2015a). Direct conversion of normal and Alzheimer’s disease human fibroblasts into neuronal cells by small molecules. Cell Stem Cell, 17(2): 204–212
Hu Z, Pu J, Jiang H, Zhong P, Qiu J, Li F, Wang X, Zhang B, Yan Z, Feng J (2015b). Generation of naivetropic induced pluripotent stem cells from Parkinson’s disease patients for high-efficiency genetic manipulation and disease modeling. Stem Cells Dev, 24(21): 2591–2604
Israel M A, Yuan S H, Bardy C, Reyna S M, Mu Y, Herrera C, Hefferan M P, van Gorp S, Nazor K L, Boscolo F S, Carson C T, Laurent L C, Marsala M, Gage F H, Remes A M, Koo E H, Goldstein L S B (2012). Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature, 482(7384): 216–220
Ittner L M, Götz J (2011). Amyloid-ß and tau—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci, 12(2): 65–72
Kim D, Kim C H, Moon J I, Chung Y G, Chang M Y, Han B S, Ko S, Yang E, Cha K Y, Lanza R, Kim K S (2009). Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell, 4(6): 472–476
Kim Y H, Choi S H, D’Avanzo C, Hebisch M, Sliwinski C, Bylykbashi E, Washicosky K J, Klee J B, Brüstle O, Tanzi R E, Kim D Y (2015). A 3D human neural cell culture system for modeling Alzheimer’s disease. Nat Protoc, 10(7): 985–1006
Kondo T, Asai M, Tsukita K, Kutoku Y, Ohsawa Y, Sunada Y, Imamura K, Egawa N, Yahata N, Okita K, Takahashi K, Asaka I, Aoi T, Watanabe A, Watanabe K, Kadoya C, Nakano R, Watanabe D, Maruyama K, Hori O, Hibino S, Choshi T, Nakahata T, Hioki H, Kaneko T, Naitoh M, Yoshikawa K, Yamawaki S, Suzuki S, Hata R, Ueno S, Seki T, Kobayashi K, Toda T, Murakami K, Irie K, Klein W L, Mori H, Asada T, Takahashi R, Iwata N, Yamanaka S, Inoue H (2013). Modeling Alzheimer’s disease with iPSCs reveals streßs phenotypes associated with intracellular Aß and differential drug responsiveness. Cell Stem Cell, 12(4): 487–496
Kyttälä A, Moraghebi R, Valensisi C, Kettunen J, Andrus C, Pasumarthy K K, Nakanishi M, Nishimura K, Ohtaka M, Weltner J, van Handel B, Parkkonen O, Sinisalo J, Jalanko A, Hawkins R D, Woods N B, Otonkoski T, Trokovic R (2016). Genetic variability overrides the impact of parental cell type and determines iPSC differentiation potential. Stem Cell Rep, 6(2): 200–212
Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, Herms S, Wernet P, Kögler G, Müller F J, Koch P, Brüstle O (2012). Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods, 9(6): 575–578
Lambert J C, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido M J, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fiévet N, Barberger-Gateau P, Engelborghs S, de Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo M M, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanché H, Dartigues J F, Tzourio C, Gut I, van Broeckhoven C, Alpérovitch A, Lathrop M, Amouyel P, the European Alzheimer’s Disease Initiative Investigators (2009). Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet, 41(10): 1094–1099
Lancaster M A, Knoblich J A (2014). Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc, 9(10): 2329–2340
Lancaster M A, Renner M, Martin C A, Wenzel D, Bicknell L S, Hurles M E, Homfray T, Penninger J M, Jackson A P, Knoblich J A (2013). Cerebral organoids model human brain development and microcephaly. Nature, 501(7467): 373–379
Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, de Vos J, Lehmann S, Lemaitre J M (2011). Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev, 25(21): 2248–2253
Lau S, Rylander Ottosson D, Jakobsson J, Parmar M (2014). Direct neural conversion from human fibroblasts using self-regulating and nonintegrating viral vectors. Cell Reports, 9(5): 1673–1680
Li W, Zhou H, Abujarour R, Zhu S, Young Joo J, Lin T, Hao E, Schöler H R, Hayek A, Ding S (2009). Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells, 27(12): 2992–3000
Lim SM, Choi WJ, Oh K W, Xue Y, Choi J Y, Kim S H, Nahm M, Kim Y E, Lee J, Noh M Y, Lee S, Hwang S, Ki C S, Fu X D, Kim S H (2016). Directly converted patient-specific induced neurons mirror the neuropathology of FUS with disrupted nuclear localization in amyotrophic lateral sclerosis. Mol Neurodegener, 11 (1): 8
Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, Lin X, Hahm H S, Hao E, Hayek A, Ding S (2009). A chemical platform for improved induction of human iPSCs. Nat Methods, 6(11): 805–808
Liras A, Segovia C, Gabán A S (eds.) (2013). Induced Pluripotent Stem Cells: Therapeutic Applications in Monogenic and Metabolic Diseases, and Regulatory and Bioethical Considerations. InTechOpen
Liu M L, Zang T, Zhang C L (2016). Direct lineage reprogramming reveals disease-specific phenotypes of motor neurons from human ALS patients. Cell Reports, 14(1): 115–128
Liu ML, Zang T, Zou Y, Chang J C, Gibson J R, Huber KM, Zhang C L (2013). Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat Commun, 4: 2183
Mahmoudi S, Brunet A (2012). Aging and reprogramming: a two-way street. Curr Opin Cell Biol, 24(6): 744–756
Marion R M, Strati K, Li H, Tejera A, Schoeftner S, Ortega S, Serrano M, Blasco M A (2009). Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell, 4(2): 141–154
Mascalchi M, Salvi F, Valzania F, Marcacci G, Bartolozzi C, Tassinari C A (1995). Corticospinal tract degeneration in motor neuron disease. AJNR Am J Neuroradiol, 16(4 Suppl): 878–880
Mertens J, Paquola A C, Ku M, Hatch E, Böhnke L, Ladjevardi S, McGrath S, Campbell B, Lee H, Herdy J R, Gonçalves J T, Toda T, Kim Y, Winkler J, Yao J, Hetzer M W, Gage F H (2015). Directly reprogrammed human neurons retain aging-associated transcriptomic signatures and reveal age-related nucleocytoplasmic defects. Cell Stem Cell, 17(6): 705–718
Miller J D, Ganat Y M, Kishinevsky S, Bowman R L, Liu B, Tu E Y, Mandal P K, Vera E, Shim J W, Kriks S, Taldone T, Fusaki N, Tomishima M J, Krainc D, Milner T A, Rossi D J, Studer L (2013). Human iPSC-based modeling of late-onset disease via progerininduced aging. Cell Stem Cell, 13(6): 691–705
Muratore C R, Rice H C, Srikanth P, Callahan D G, Shin T, Benjamin L N, Walsh D M, Selkoe D J, Young-Pearse T L (2014). The familial Alzheimer’s disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum Mol Genet, 23(13): 3523–3536
Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S (2008). Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol, 26(1): 101–106
Narsinh K H, Sun N, Sanchez-Freire V, Lee A S, Almeida P, Hu S, Jan T, Wilson K D, Leong D, Rosenberg J, Yao M, Robbins R C, Wu J C (2011). Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest, 121(3): 1217–1221
Ohta E, Nihira T, Uchino A, Imaizumi Y, Okada Y, Akamatsu W, Takahashi K, Hayakawa H, Nagai M, Ohyama M, Ryo M, Ogino M, Murayama S, Takashima A, Nishiyama K, Mizuno Y, Mochizuki H, Obata F, Okano H 2015. I2020T mutant LRRK2 iPSC-derived neurons in the Sagamihara family exhibit increased Tau phosphorylation through the AKT/GSK-3 signaling pathway. Human Mol Genet, 24(17):4879–4900
Okita K, Ichisaka T, Yamanaka S (2007). Generation of germlinecompetent induced pluripotent stem cells. Nature, 448(7151): 313–317
Ooi L, Sidhu K, Poljak A, Sutherland G, O’Connor M D, Sachdev P, Münch G (2013). Induced pluripotent stem cells as tools for disease modelling and drug discovery in Alzheimer’s disease. J Neural Transm (Vienna), 120(1): 103–111
Pang Z P, Yang N, Vierbuchen T, Ostermeier A, Fuentes D R, Yang T Q, Citri A, Sebastiano V, Marro S, Südhof T C, Wernig M (2011). Induction of human neuronal cells by defined transcription factors. Nature, 476(7359): 220–223
Pasinelli P, Brown R H (2006). Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci, 7(9): 710–723
Price J L, Ko A I, Wade MJ, Tsou S K, McKeel DW, Morris J C (2001). Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol, 58(9): 1395–1402
Ring K L, An M C, Zhang N, O’Brien R N, Ramos EM, Gao F, Atwood R, Bailus B J, Melov S, Mooney S D, Coppola G, Ellerby L M, the RING (2015). Genomic analysis reveals disruption of striatal neuronal development and therapeutic targets in human Hungtinton’s disease neural stem cells. Stem Cell Rep, 5(6): 1023–1038
Ring K L, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang W R, Kreitzer A C, Huang Y (2012). Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell, 11(1): 100–109
Ryan S D, Dolatabadi N, Chan S F, Zhang X, Akhtar M W, Parker J, Soldner F, Sunico C R, Nagar S, Talantova M, Lee B, Lopez K, Nutter A, Shan B, Molokanova E, Zhang Y, Han X, Nakamura T, Masliah E, Yates J R, Nakanishi N, Andreyev A Y, Okamoto S, Jaenisch R, Ambasudhan R, Lipton S A (2013). Isogenic human iPSC Parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-PGC1a transcription. Cell, 155(6): 1351–1364
Schuster J, Halvardson J, Pilar Lorenzo L, Ameur A, Sobol M, Raykova D, Annerén G, Feuk L, Dahl N (2015). Transcriptome profiling reveals degree of variability in induced pluripotent stem cell lines: Impact for human disease modeling. Cell Reprogram, 17(5): 327–337
Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D (2011). Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci, 31 (16): 5970–5976
Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A (2008). Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol, 6 (10): e253
Soldner F, Hockemeyer D, Beard C, Gao Q, Bell G W, Cook E G, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O, Jaenisch R (2009). Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell, 136(5): 964–977
Soldner F, Laganière J, Cheng A W, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe L I, Myers R H, Lindquist S, Zhang L, Guschin D, Fong L K, Vu B J, Meng X, Urnov F D, Rebar E J, Gregory P D, Zhang H S, Jaenisch R (2011). Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell, 146(2): 318–331
Sommer C A, Christodoulou C, Gianotti-Sommer A, Shen S S, Sailaja B S, Hezroni H, Spira A, Meshorer E, Kotton D N, Mostoslavsky G (2012). Residual expression of reprogramming factors affects the transcriptional program and epigenetic signatures of induced pluripotent stem cells. PLoS ONE, 7 (12): e51711
Sproul A, Jacob S, Paquet D, Ortiz-Virumbrales M, Campos B, Gandy S, Tessier-Lavigne M, Noggle S (2014). Using familial Alzheimer’s disease and isogenic control IPSc-derived basal forebrain neurons to model AD. Alzheimers Dement, 10 (4): 643–P644
Strong M J, Yang W (2011). The frontotemporal syndromes of ALS. Clinicopathological correlates. J Mol Neurosci, 45(3): 648–655
Su Y, Blazey T M, Owen C J, Christensen J J, Friedrichsen K, Joseph-Mathurin N, Wang Q, Hornbeck R C, Ances B M, Snyder A Z, Cash L A, Koeppe R A, Klunk W E, Galasko D, Brickman A M, McDade E, Ringman JM, Thompson PM, Saykin A J, Ghetti B, Sperling R A, Johnson K A, Salloway S P, Schofield P R, Masters C L, Villemagne V L, Fox N C, Förster S, Chen K, Reiman E M, Xiong C, Marcus D S, Weiner M W, Morris J C, Bateman R J, Benzinger T L, the Dominantly Inherited Alzheimer Network (2016). Quantitative amyloid imaging in autosomal dominant Alzheimer’s disease: Results from the DIAN study group. PLoS ONE, 11 (3): e0152082
Suhr S T, Chang E A, Tjong J, Alcasid N, Perkins G A, Goissis M D, Ellisman M H, Perez G I, Cibelli J B (2010). Mitochondrial rejuvenation after induced pluripotency. PLoS ONE, 5 (11): e14095
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5): 861–872
Tanzi R E, Bertram L (2005). Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell, 120(4): 545–555
Thatava T, Kudva Y C, Edukulla R, Squillace K, de Lamo J G, Khan Y K, Sakuma T, Ohmine S, Terzic A, Ikeda Y (2013). Intrapatient variations in type 1 diabetes-specific iPS cell differentiation into insulin-producing cells. Mol Ther, 21(1): 228–239
The Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) (1998). Cognitive function and dementia in six areas of England and Wales: the distribution of MMSE and prevalence of GMS organicity level in the MRC CFA Study. Psychol Med, 28(2): 319–335
Tsai M S, Tangalos E G, Petersen R C, Smith G E, Schaid D J, Kokmen E, Ivnik R J, Thibodeau S N (1994). Apolipoprotein E: risk factor for Alzheimer disease. Am J Hum Genet, 54(4): 643–649
Vera E, Studer L (2015). When rejuvenation is a problem: challenges of modeling late-onset neurodegenerative disease. Development, 142 (18): 3085–3089
Vierbuchen T, Ostermeier A, Pang Z P, Kokubu Y, Südhof T C, Wernig M (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature, 463(7284): 1035–1041
Wapinski O L, Vierbuchen T, Qu K, Lee Q Y, Chanda S, Fuentes D R, Giresi P G, Ng Y H, Marro S, Neff N F, Drechsel D, Martynoga B, Castro D S, Webb A E, Südhof T C, Brunet A, Guillemot F, Chang H Y, Wernig M (2013). Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell, 155(3): 621–635
West MJ, Coleman P D, Flood D G, Troncoso J C (1994). Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet, 344(8925): 769–772
Wilcock DM (2010). The usefulness and challenges of transgenic mouse models in the study of Alzheimer’s disease. CNS Neurol Disord Drug Targets, 9(4): 386–394
Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Yoshizaki T, Yamanaka S, Okano H, Suzuki N (2011). Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum Mol Genet, 20(23): 4530–4539
Yoo A S, Sun A X, Li L, Shcheglovitov A, Portmann T, Li Y, Lee- Messer C, Dolmetsch R E, Tsien R W, Crabtree G R (2011). MicroRNA-mediated conversion of human fibroblasts to neurons. Nature, 476(7359): 228–231
Zhou W, Freed C R (2009). Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells, 27 (11): 2667–2674
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributed equally to the work
Rights and permissions
About this article
Cite this article
Truong, A., Si, E., Duncan, T. et al. Modeling neurodegenerative disorders in adult somatic cells: A critical review. Front. Biol. 11, 232–245 (2016). https://doi.org/10.1007/s11515-016-1413-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-016-1413-3