Abstract
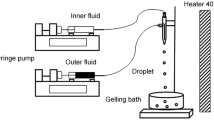
A new droplets millifluidic/inverse gelation based process was used to produce core-shell alginate milli-capsules. Water-in-oil (W/O) emulsion dispersed phase containing Ca2+ ions was directly injected into a continuous alginate phase to generate a secondary W/O/W emulsion. Due to the cross-linking of alginate molecules by Ca2+ ions release, core-shell milli-capsules were formed with a very high oil loading. The influence of the curing time and of the storage conditions on capsules physico-chemical properties were investigated. It was first found as expected that alginate membrane thickness increased with curing time in the collecting bath. However, a plateau was reached for the higher curing times, in close relation with previous observations (Martins, Poncelet, Marquis, Davy, & Renard, 2017b) that an external oil layer surrounded the surface of W/O emulsion drops that acted as a barrier and hindered the release of aqueous CaCl2 droplets during curing time. Compression experiments on individual capsules revealed that alginate membrane thickness was inversely related to its mechanical properties, i.e. the thicker membrane, the lower surface Young modulus. Surface Young modulus ranged from 61 to 26 N/m at curing times of 3 and 45 min, respectively. This result was explained in terms of enhanced swelling properties of alginate membrane with curing time or storage conditions. Drying capsules led to much more resistant membranes due to the loss of water. Oil loading of 80 wt% was obtained for dry capsules whatever the conditions used.









Similar content being viewed by others
References
B. Lupo, A. Maestro, M. Porras, J.M. Gutiérrez, C. González, Preparation of alginate microspheres by emulsification/internal gelation to encapsulate cocoa polyphenols. Food Hydrocoll. 38, 56–65 (2014)
E. Martins, D. Renard, J. Davy, M. Marquis, D. Poncelet, Oil core microcapsules by inverse gelation technique. J. Microencapsul. 32(1), 86–95 (2015)
S.J. Risch, G.A.A. Reineccius, Flavor Encapsulation, ACS SymposiumSeries 370 (American Chemical Society, Washington, DC, 1988)
M. Jin, Y. Zheng, Q. Hu, Preparation and characterization of bovine serum albumin alginate/chitosan microspheres for oral administration. Asian J. Pharm. Sci. 4(4), 215–220 (2009)
K. Ziani, Y. Fang, D.J. McClements, Encapsulation of functional lipophilic components in surfactant-based colloidal delivery systems: vitamin E, vitamin D, and lemon oil. Food Chem. 134(2), 1106–1112 (2012)
C. Ouwerx, N. Velings, M.M. Mestdagh, M.A.V. Axelos, Physico-chemical properties and rheology of alginate gel beads formed with various divalent cations. Polymer Gels and Networks 6(5), 393–408 (1998)
D. Poncelet, V. Babak, C. Dulieu, A. Picot, A physico-chemical approach to production of alginate beads by emulsification-internal ionotropic gelation. Colloids Surf. A Physicochem. Eng. Asp. 155(2–3), 171–176 (1999)
D. Quong, R.J. Neufeld, G. Skjåk-Bræk, D. Poncelet, External versus internal source of calcium during the gelation of alginate beads for DNA encapsulation. Biotechnol. Bioeng. 57(4), 438–446 (1998)
S. Abang, E.S. Chan, D. Poncelet, Effects of process variables on the encapsulation of oil in ca-alginate capsules using an inverse gelation technique. J. Microencapsul. 29(5), 417–428 (2012)
E. Martins, D. Poncelet, D. Renard, A novel method of oil encapsulation in core-shell alginate microcapsules by dispersion-inverse gelation technique. React. Funct. Polym. 114, 49–57 (2017a)
E. Martins, D. Poncelet, M. Marquis, J. Davy, D. Renard, Monodisperse core-shell alginate (micro)-capsules with oil core generated from droplets millifluidic. Food Hydrocoll. 63, 447–456 (2017b)
J.-Y. Wang, Y. Jin, R. Xie, J.-Y. Liu, X.-J. Ju, T. Meng, L.-Y. Chu, Novel calcium-alginate capsules with aqueous core and thermo-responsive membrane. J. Colloid Interface Sci. 353, 61–68 (2011)
A. Schmit, L. Courbin, M. Marquis, D. Renard, P. Panizza, A pendant drop method for the production of calibrated double emulsions and emulsion gels. Rsc Advances 4(54), 28,504–28,510 (2014)
M. Rachik, D. Barthes-Biesel, M. Carin, F. Edwards-Levy, Identification of the elastic properties of an artificial capsule membrane with the compression test: effect of thickness. J. Colloid Interface Sci. 301(1), 217–226 (2006)
A. Fery, R. Weinkamer, Mechanical properties of micro-and nanocapsules: Single-capsule measurements. Polymer 48(25), 7221–7235 (2007)
A.M. Al-Sabagh, The relevance HLB of surfactants on the stability of asphalt emulsion. Colloids Surf. A Physicochem. Eng. Asp. 204(1–3), 73–83 (2002)
A.L. Márquez, A. Medrano, L.A. Panizzolo, J.R. Wagner, Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. J. Colloid Interface Sci. 341(1), 101–108 (2010)
J.H. Su, J. Flanagan, Y. Hemar, H. Singh, Synergistic effects of polyglycerol ester of polyricinoleic acid and sodium caseinate on the stabilisation of water-oil-water emulsions. Food Hydrocoll. 20(2–3), 261–268 (2006)
K.S. Karunadasa, C.H. Manoratne, H.M.T.G.A. Pitawala, R.M.G. Rajapakse, Relative stability of hydrated/anhydrous products of calcium chloride during complete dehydration as examined by high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 120, 167–172 (2018)
A. Souza, J.C. Santos, M.M. Conceição, M.C. Silva, S. Prasad, A thermoanalytic and kinetic study of sunflower oil. Braz. J. Chem. Eng. 21(2), 265–273 (2004)
Zhao, Y., Huang, Z., Zhang, J., Wu, W., Wang, M., & Fan, L. (2010). Thermal Degradation of Sodium Alginate-Incorporated Soy Protein Isolate/Glycerol Composite Membranes.
J.P. Soares, J.E. Santos, G.O. Chierice, E.T.G. Cavalheiro, Thermal behavior of alginic acid and its sodium salt. Eclética Química 29(2), 57–64 (2004)
A.K. Pawlik, Duplex emulsions for healthy foods (Doctoral dissertation, University of Birmingham, 2012)
A. Gray, S. Egan, S. Bakalis, Z. Zhang, Determination of microcapsule physicochemical, structural, and mechanical properties. Particuology 24, 32–43 (2016)
S. Leick, S. Henning, P. Degen, D. Suter, H. Rehage, Deformation of liquid-filled calcium alginate capsules in a spinning drop apparatus. Phys. Chem. Chem. Phys. 12(12), 2950–2958 (2010)
A. Blandino, M. Macias, D. Cantero, Formation of calcium alginate gel capsules: influence of sodium alginate and CaCl2 concentration on gelation kinetics. J. Biosci. Bioeng. 88(6), 686–689 (1999)
E. Martins, D. Poncelet, C.R. Ramires, D. Renard, Oil encapsulation techniques using alginate as encapsulating agent: Applications and drawbacks. J. Microencapsul. 34(8), 754–771 (2017c)
M. Briššová, I. Lacík, A.C. Powers, A.V. Anilkumar, T. Wang, Control and measurement of permeability for design of microcapsule cell delivery system. J. Biomed. Mater. Res. 39(1), 61–70 (1998)
M.P. Neubauer, M. Poehlmann, A. Fery, Microcapsule mechanics: From stability to function. Adv. Colloid Interf. Sci. 207, 65–80 (2014)
E.S. Chan, T.K. Lim, W.P. Voo, R. Pogaku, B.T. Tey, Z. Zhang, Effect of formulation of alginate beads on their mechanical behavior and stiffness. Particuology 9(3), 228–234 (2011)
Z. Marcadé-Prieto, Zhang. Mechanical characterization of microspheres - capsules, cells and microspheres: A review. J. Microencapsul. 29(3), 277–285 (2012)
M. Lekka, D. Sainz-Serp, A.J. Kulik, C. Wandrey, Hydrogel Microspheres: Influence of Chemical Composition on Surface Morphology, Local Elastic Properties, and Bulk Mechanical Characteristics. Langmuir 20, 9968–9977 (2004)
M. Carin, D. Barthès-Biesel, F. Edwards-Lévy, C. Postel, D.C. Andrei, Compression of biocompatible liquid-filled HSA-alginate capsules: Determination of the membrane mechanical properties. Biotechnol. Bioeng. 82(2), 207–212 (2003)
M.W. Keller, N.R. Sottos, Mechanical properties of microcapsules used in a self-healing polymer. Exp. Mech. 46(6), 725–733 (2006)
S. Leick, A. Kemper, H. Rehage, Alginate/poly-L-lysine capsules: mechanical properties and drug releasecharacteristics. Soft Matter 7, 6684–6694 (2011)
G.B. Messaoud, L. Sánchez-González, A. Jacquot, L. Probst, S. Desobry, Alginate/sodium caseinate aqueous-core capsules: A pH-responsive matrix. J. Colloid Interface Sci. 440, 1–8 (2015)
S. Leick, M. Kott, P. Degen, S. Henning, T. Päsler, D. Suter, H. Rehage, Mechanical properties of liquid-filled shellac composite capsules. Phys. Chem. Chem. Phys. 13(7), 2765–2773 (2011)
E. Zwar, A. Kemna, L. Richter, P. Degen, H. Rehage, Production, deformation and mechanical investigation of magnetic alginate capsules. J. Phys. Condens. Matter 30(8) (2018) number 085101
P. Lopez-Sanchez, N. Fredriksson, A. Larsson, A. Altskärc, A. Strömb, High sugar content impacts microstructure, mechanics and release of calcium-alginate gels. Food Hydrocoll. 84, 26–33 (2018)
P.E. Ramos, P. Silva, M.M. Alario, L.M. Pastrana, J.A. Teixeira, M.A. Cerqueira, A.A. Vicente, Effect of alginate molecular weight and M/G ratio in beads properties foreseeing the protection of probiotics. Food Hydrocoll. 77, 8–16 (2018)
Acknowledgements
We kindly acknowledge the supply of PGPR 90 by Danisco (France), the supply of alginate by Cargill (France), the technical assistance of Jean-Eudes Megret for compression experiments, and the financial support of one author (Mariana Pereda) by the National Research Council of Republic Argentina (CONICET) through the program “Becas en el Exterior para Jóvenes Investigadores del CONICET” (Argentina).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 494 kb)
Rights and permissions
About this article
Cite this article
Pereda, M., Poncelet, D. & Renard, D. Characterization of Core-Shell Alginate Capsules. Food Biophysics 14, 467–478 (2019). https://doi.org/10.1007/s11483-019-09595-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-019-09595-x