Abstract
Neurological complications of human immunodeficiency virus (HIV) infection are a public health problem despite the availability of active antiretroviral therapies. The neuropathogenesis of HIV infection revolves around a complex cascade of events that include viral infection and glial immune activation, monocyte–macrophage brain infiltration, and secretion of a host of viral and cellular inflammatory and neurotoxic molecules. Although there is evidence that HIV-infected drug abusers experience more severe neurological disease, the biological basis for this finding is unknown. A scientific workshop organized by the National Institute on Drug Abuse (NIDA) was held on March 23–24, 2006 to address this question. The goal of the meeting was to bring together basic science and clinical researchers who are experts in NeuroAIDS, glial immunity, drugs of abuse, and/or pharmacology in order to find new approaches to understanding interactions between drug abuse and neuroAIDS. The format of the meeting was designed to stimulate open discussion and forge new multidisciplinary research collaborations. This report includes transcripts of active discussions and short presentations from invited participants. The presentations were separated into sections that included: Glial Biology, Inflammation, and HIV; Pharmacology, Neurotoxicology, and Neuroprotection; NeuroAIDS and Virology; and Virus–Drug and Immune–Drug Interactions. Research priorities were identified. Additional information about this meeting is available through links from the NIDA AIDS Research Program website (http://www.nida.nih.gov/about/organization/arp/arp-websites.htm).
Similar content being viewed by others
Introduction
Although the incidence of HIV-associated dementia (HAD) has declined since the advent of modern antiretroviral therapies, the prevalence of neurological disease continues to increase as HIV-infected patients are living longer. Intravenous drug use accounts for nearly one-quarter of new acquired immune deficiency syndrome (AIDS) cases in the United States, and opioid pain medications with addictive properties are commonly used to treat disease complications, such as peripheral neuropathy. There is recent evidence that demonstrates a relationship between drug use and more severe disease manifestations including HIV-associated cognitive impairments. However, the biological basis for this relationship is unknown. It may be that drug use affects viral entry into the central nervous system (CNS), changes the neural environment in a way that affects viral replication or evolution, stimulates events that contribute to disease progression in the brain, or alters the susceptibility of neural cells to damage from HIV infection.
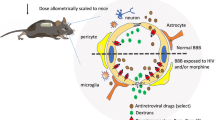
Recent research has begun to address the biological impact of drugs of abuse on HIV-associated neurologic disease, but these efforts have been limited by the complexities of human studies of HIV-infected drug users, the high costs of simian immunodeficiency virus (SIV) studies in nonhuman primates, and the lack of an ideal small animal or laboratory approach systems that sufficiently model HIV/AIDS, and particularly neurological disease, as it occurs in the infected human host. An interactive workshop to address this need for interdisciplinary NeuroAIDS research was held on March 23–24, 2006, in Bethesda, MD. The workshop, sponsored by the National Institute on Drug Abuse (NIDA) AIDS Research Program, brought together a small group of scientists to actively discuss specific research goals related to the interactions of addictive drugs and HIV neuropathogenesis, including glial inflammation (Fig. 1). Integration of current thinking from multiple basic and clinical research perspectives was emphasized and included priorities for future research. To access the slide presentations referenced by individual speakers in these transcripts, please see the on-line Meeting Reports link from the NIDA AIDS Research Program website (http://www.nida.nih.gov/about/organization/arp/arp-websites.htm).
Diagram showing how integration of research areas may permit interdisciplinary approaches to understanding the mechanisms of HIV/AIDS neuropathogenesis in the context of drug abuse, glial activation, and inflammation (courtesy of NIDA http://www.csrideas.com/nida/neuroconf/index.htm).
Overview of NeuroAIDS, drug abuse, and inflammation
A. Nath
My talk is divided into three components: (1) interactions of the virus and the brain; (2) interactions of drugs of abuse with the brain; and (3) effects of drugs of abuse on viral life cycle in the brain. I have termed these interactions between the brain, drugs of abuse and HIV the “pleasure triangle,” because the brain is the seat of pleasure, drugs of abuse are often natural products taken to derive pleasure, and HIV transmission occurs while seeking pleasure.
The first part of the triangle deals with interactions of HIV with the brain. Viruses have existed in our environment for a very long time and are the simplest of all organisms. However, our understanding of the interactions between the virus and its host, man, is incomplete. If one looks at a virus, it is nothing more than a strand of nucleic acid and some protein surrounding it. Yet as the battle between virus and man continues, the virus often survives and man dies.
The interaction between retroviruses and humans is not anything new. Approximately 11% of the human genome contains retroviral sequences. It is possible that we have been infected with related retroviruses over a long period of time, and once they get integrated into our genome they get transmitted genetically. Maybe that has, in part, contributed to our own evolution. All retroviruses have two long terminal repeats (LTR), a group-specific antigen (Gag), polymerase (Pol) and envelope (Env) genes. If one examines the human genome, one will find pieces of retroviral gene sequences separated by introns. Several of us assembled here today have been working on these human and/or retroviral sequences and studying how they affect disease pathogenesis.
Human retroviruses commonly affect the CNS. Indeed, human T cell lymphotropic virus type 1 (HTLV-1) can cause a myelopathy and neuropathy, HTLV-2 can also cause a myelopathy, and the human immunodeficiency viruses type 1 and 2 (HIV-1 and HIV-2) can cause dementia, myelopathy, and neuropathy. HIV affects the CNS by causing cognitive, motor, and behavior dysfunction. Among the nuclei within the basal ganglia, pathological abnormalities including multinucleated giant cells, microglial nodules, and HIV-infected microglial cells and infiltrating macrophages, are most common within the putamen and caudate (Navia et al. 1986). Molecular studies confirm that viral load is also maximal in this region (Fujimura et al. 1997). Interestingly, significant neuronal cell loss also has been noted in the pars compacta of the substantia nigra. The remaining cell bodies are more heavily pigmented and shrunken in size (Reyes et al. 1991). Extrapolation of data presented in this manuscript suggests that these changes are most prominent in intravenous drug abusers. Despite these prominent neuronal changes, there was no evidence of multinucleated giant cells, microglial nodules, or HIV-infected cells in the substantia nigra of these patients (Reyes et al. 1991). Thus the effects of viral infection in the brain are more widespread than the infection itself.
Shown here is a magnetic resonance imaging (MRI) brain scan of a 30-year-old methamphetamine-abusing HIV-1-infected person that I had followed in the clinic. This scan shows massive atrophy in the basal ganglia and frontal lobes. One can often see profound changes in the brain in some of these infected patients who are also drug abusers. If one gives fluorodeoxyglucose to an HIV-infected patient, one can demonstrate that the earliest brain areas that are involved are the basal ganglia. I think this is important as drugs of abuse also affect the same areas, so interactions between virus and drugs are potentially of great interest. In the era of highly active antiretroviral treatment (HAART), the clinical presentation has changed significantly. Previously, before HAART was available, the progression of dementia was rapid and could lead to death within a matter of months. However, with the advent of HAART, some patients can actually get better, some may not change at all, and others proceed in a chronic active form that we are seeing much of these days.
Pathologically, the virus is present predominantly in perivascular macrophages and microglial cells and also in astrocytes, where it may become latent. However, productive viral replication is predominantly in the macrophage lineage cells. HIV-1 gp120 immunostaining shows that macrophages and microglia express the envelope protein in single cells and in multinucleated giant cells. The multinucleated giant cells are nothing else but fused macrophages. Microglial nodules are composed of some macrophages and microglia but lymphocytes are present as well. Significant astrocytosis and microglial activation also occur in these patients. With amyloid precursor protein (APP) staining, one can see that the axons themselves have these small areas of beading as though the axonal flow is interrupted and they have become “constipated.” There is dendritic loss together with neuronal loss that Dr. Eliezer Masliah and other groups have shown elegantly in a series of studies. However, these neurons are typically not infected so the neuronal loss and neuronal injury are indirectly mediated (Everall et al. 1995, 1999; Masliah et al. 1992, 1997). I think this concept is important for studying interactions with drugs of abuse with HIV, because these drugs, although potentially toxic themselves, are being used in an environment where the HIV-infected cell is producing products that are toxic to neurons. Thus the effects of the two could get compounded (Nath et al. 2002).
Dr. Christopher Power’s work shows that all viruses are not the same when they enter the brain and that the virus evolves within the nervous system. In his studies, he showed clustering of envelope sequences isolated from the brains of patients with HIV dementia, suggesting that it is possible that some of these unique viral signatures present in the brain might contribute to unique pathophysiological process (van Marle and Power 2005).
HIV predominantly affects the immune cells, and it is very interesting that it infects the very cells that are important in controlling viral replication. So the virus in that sense is smart. It disables the adaptive immune system, which is, in part, the CD4+ T lymphocyte. It also affects the innate immune system that includes the macrophage and astrocytes within the brain (Fig. 2). Therefore, viral immune control is difficult to achieve and that contributes to HIV survival within the brain and elsewhere. Clearly, once the adaptive immune system is impaired, the body uses other mechanisms for pathogen control. The host stimulates the innate immune response, the primitive immune response that is the major defense mechanism in invertebrates and that consists of nitric oxide (NO), free radicals, proteases, and possibly cytokines as well (Fig. 3). That is what you see in patients with HIV dementia; although they are losing their cellular immune responses, their innate responses are increasing. This results in nonspecific immune responses, neuronal loss, and glial cell activation (McArthur et al. 2005).
Stimulation of innate immunity parallels loss of adaptive immune responses. While the adaptive immune response evolved in vertebrate animals, the innate immune response is the major defense mechanism in the invertebrates. The innate immune response includes the production of a number or nonspecific substances such as nitric oxide, free radicals, proteases, and cytokines. In HIV infection, these innate immune responses get stimulated in the brain later in the course of the illness. Although these immune responses may impact the virus, because of their nonspecific nature, they may also damage the host. The neurons are particularly vulnerable to such insults.
The second part of the triangle involves “interactions of drugs of abuse with the brain.” The purpose of the nervous system is to “seek pleasure and avoid pain.” This is true even in the most primitive of all neural systems. For example, a starfish does not really have an organized nervous system, but its nerves project to the periphery and it responds to noxious stimuli and will retract to avoid them. But along the evolutionary chain, as soon as the brain is formed, you now have the ability to seek pleasure. An insect has a small brain and a comparatively large peripheral nervous system. As an organism becomes more and more complex, the size of the brain increases and its ability to interact with its surroundings and environment and the ability to gain pleasure evolves.
We derive pleasure from all of our five senses: vision, hearing, touch, smell, and taste. All our pleasurable experiences don’t always translate to addiction. Although you might enjoy watching the setting sun, you don’t get addicted to it. When does pleasure translate to addiction and how does that occur? One possibility is that once we try to derive pleasure by driving the same pathways through chemical exposure we become extremely prone to developing addiction. This would explain the chemical addiction that we most commonly see. There are multiple such drugs that we know of and together they form the drugs of abuse. But the problem in studying addiction is that the person on the street doesn’t just take pure cocaine or just take methamphetamine. He is taking all of these things, whichever is the flavor of the month, or whatever he can afford that day, or whatever is available in society. Another problem is that these drugs are not pure. When we design our experiments, most often we want the purest compounds to study, but what the patients are taking is anything but pure. So that poses an important challenge to us, as to how do we really study these things that are going to make a difference to the epidemic? Nevertheless, these drugs affect multiple neuronal and glial systems, the immune system, and probably affect viral replication as well. They have a multitude of effects on the body.
The innate immune system is the one that is found in invertebrates and as evolution occurs, the nervous system and then the adaptive immune system form. The nervous system and the adaptive immune system share much in common. You will find opiate receptors, elements of the dopaminergic system, cannanabinoid receptors and glutamate in both. What we typically think of as elements and receptors within the CNS are also present in the immune system, so you can find these on lymphocytes and macrophages. It is thus not surprising that these drugs of abuse will affect both the CNS and immune systems. The drugs are abused because of their effects on the CNS, yet they also interact with the immune system. If the immune system is already failing during HIV infection, then drugs of abuse could potentially have a more profound effect.
Both HIV and some illicit drugs affect the basal ganglia and hence the dopaminergic system may be affected (Nath et al. 2000). Some patients may have very profound effects. Shown here is an MRI scan of a patient with HIV infection who abused cocaine. Profound MRI changes can be seen in the basal ganglia, the surrounding white matter, and with concomitant neuronal loss and spongiform changes seen histopathologically. The next slide shows patients with HIV-1 infection with or without encephalitis and drug abuse. With the combination of drug abuse and HIV infection, there is a profound loss of neuronal staining in the dentate gyrus, which is an area controlling learning and memory.
The third part of the triangle asks the question of whether there can be interactions between drugs of abuse and HIV replication. This is an area that needs to be studied more extensively, and what we have right now is phenomenology. We really don’t have mechanisms for this. I wanted to show you this slide from published work of Dr. Michael Podell. He first showed that methamphetamine causes massive up-regulation of FIV production in cats, and he further studied it specifically in astrocytes and demonstrated 10- to 20-fold increases in viral replication. The data show clearly that with an increasing dose of methamphetamine, there is induction of FIV replication (Gavrilin et al. 2002).
Dr. Milan Fiala showed that if you put cocaine on endothelial cells, you find that it increases viral entry via disruption of the blood–brain barrier (Fiala et al. 2005). We looked at cocaine for LTR transactivation in an astrocyte cell line, and what we found was that in a dose-responsive manner we see transactivation of the viral LTR.
There is relatively more literature on this topic with morphine as compared to the other drugs, and Dr. Phillip Peterson’s group has shown that morphine can induce CCR5 as well as cause up-regulation of HIV in macrophages and microglial cells (Peterson et al. 1999). Morphine can also interact with lymphocytes, and work performed by Dr. Kurt Hauser showed that morphine could induce monocyte chemoattractant protein-1 (MCP-1/CCL2) production in conjunction with HIV-Tat protein, thus increasing macrophage trafficking into the brain (El-Hage et al. 2005).
These are the many ways in which these three factors (brain, drugs of abuse, and HIV) can interact with one another, but at the moment we are only scratching the surface. We really don’t understand all the mechanisms of interactions and we don’t understand what the consequences of these interactions might be. So I put together a few challenges that we face as researchers, in the form of a “Top Ten” countdown, but mine starts with number nine (Table 1).
(9) Drug abuse pathogenesis studies need to include glial cells and progenitor cells. Glial cells have been studied a fair bit in context with HIV infection; but when you look at drug abuse, it has always gotten the stepchild treatment and its effects of glial cells are poorly understood. The progenitor cells are the new kid on the block, so one would think there should be a lot of research coming out on the effect of HIV and drugs of abuse on progenitor cells. Although that might be the case, interestingly, in the current literature there is not much at all on this topic. This is a slide taken from the work of Drs. Diane Lawrence and Eugene Major, in which they show that human neural progenitor cells express CXCR4, so they have the ability to interact with HIV-1 gp120. They also show that these progenitor cells get infected with HIV and can produce virus (Lawrence et al. 2004).
(8) To change our mindset that research should always be hypothesis-driven. We need to start thinking about discovery-based research. Modern technology allows us to cast a wide net such that a large number of genes and proteins can be simultaneously studied, making such discovery-based research feasible. For example, as shown in this slide, using 1D gel analysis we were unable to show any differences in protein expression between Tat- and methamphetamine-treated neurons. But by 2D gel analysis, which allows the detection of a few hundred proteins simultaneously, we found that in neurons treated with Tat and methamphetamine there were three interesting proteins that were deregulated. However, when we analyzed the same proteins by mass spectroscopy, which allows the detection of thousands of proteins simultaneously, in neurons treated with methamphetamine, we found a complex of proteins being expressed that we did not see in the Tat-treated cells.
(7) To identify surrogate markers for determining who is at risk for developing neurological complications. We know that all patients exposed to HIV or drugs of abuse don’t always develop neurological consequences, only a subpopulation does. So identifying those individuals at risk is critical for these kinds of studies. Although it would be ideal to have a serum marker, I think the field would settle for a CSF, or genetic, or radiological marker, or combination thereof.
(6) Are there genetic factors that might predispose one to neurodegeneration with HIV and drug abuse? In the HIV field, at least, we have spent a fair bit of effort looking at cytokine and chemokine polymorphisms. I haven’t listed them all here. We have not yet looked at neural susceptibility genes. If we are going to look at interactions of drug abuse and HIV, those latter genes might become very important, because we are looking at the vulnerability of the neuron to neurotoxicity by products released from HIV-infected cells and the drugs of abuse. The innate vulnerability of the neuron to withstand such insults could be an important determining factor in its ultimate outcome. Some work has been carried out with APOE, but there are numerous polymorphisms in other neuronal susceptibility genes that have been reported in the literature, although have not been studied in the context of HIV infection.
This slide is taken from the literature to show that patients who have this mutation in the promoter region of MCP-1/CCL2 are more likely to develop HIV dementia. These polymorphisms vary depending on the population that you look at, e.g., Hispanics, African Americans, and European Americans (Gonzalez et al. 2002). In the next slide, we show that APOE polymorphisms also determine vulnerability to HIV dementia (Turchan-Cholewo et al. 2006). This is an example of a neural susceptibility gene marker. We have shown that in autopsy tissue of patients with HIV infection, the frontal cortex of patients with the APOE-4 allele tends to have more oxidative stress products compared to those with APOE-3. Moreover, in vitro, when human neurons with APOE-4 allele are exposed to Tat plus morphine, we see much greater toxicity compared to neurons with APOE-3 or APOE-2 alleles. In contrast, tumor necrosis factor (TNF)-induced neurotoxicity is not APOE-dependent. Thus, it depends on the nature of the toxin, and one particular susceptibility gene may not determine susceptibility to every environmental agent.
(5) To develop biologically relevant models. There will be some presentations here today discussing some of these models. I think a small animal model is desperately needed. Developing relevant animal models for drug abuse and HIV together poses a lot of challenges, because the effects of acute intoxication might be very different from those of chronic intoxication that could also be very different from drug withdrawal affects. Developing models of these kinds of things in animals and in vitro is very challenging. Ideally, we should also study exposure to multiple drugs. So the permutations and combinations of the experimental designs are very large.
(4) To develop new imaging techniques. We will have two discussions on imaging, and I threw this up as just a couple of things to think about. The Pittsburgh group has published new imaging techniques for detecting and quantifying amyloid in the brain (Klunk et al. 2004). There are new ideas being developed constantly. I think we need to start thinking of these novel techniques and how we can incorporate them into our research related to HIV and drug abuse.
(3) To develop novel therapeutic approaches. When you move onto development of therapeutics, you face huge roadblocks. Large pharmaceutical companies are not interested in the drug-abusing, HIV-infected people because they are underinsured and these populations don’t have adequate financial resources. Universities are not set up to address these issues or to be able to develop therapeutic agents in a manner that industry is capable of doing. So, for all diseases that affect the poor and impoverished, we really don’t have a good mechanism for developing drugs. I think the universities need to move into some of that drug development area.
(2) To rethink the way we conduct clinical trials for HIV dementia. If you look at clinical trials that we have carried out so far for HIV dementia, per se they have largely been failures. We really need new strategies for developing neuroprotective agents and we need to be able to do clinical trials with small sample sizes. Identifying the populations of patients that are vulnerable is going to be very critical to be able to do these kinds of clinical trials. We also need to compress the time frame from when we conduct these trials to when we report them. For example, we have studied only a handful of agents so far in phase 2 studies and to date, we haven’t done a single phase 3 study in HIV patients, and none of these drugs have shown any dramatic effect. The reporting lag—from the time they are conducted to the time they are reported—is very large and has spanned several years in most studies.
(1) Drug abuse should now be an inclusion criterion in our studies and not an exclusion criterion. Not a single clinical trial to date has been conducted on HIV-infected drug-abusing patients. In most of our clinical trials, the first thing we do is exclude the drug abusers because they are a statistical nightmare. We need to develop new ways to handle these statistical issues as they pose a big challenge for us.
Discussion following overview
H. Fox
I appreciate that the innate immune system is ancient, which it is, but remember that the innate immune system sets up an adaptive immune system, and that innate cells also have receptors from many of the neuroactive agents. Charles Janeway brought innate immunity back into prominence, and from his and works of others, it has become clear that one should not strictly separate innate and adaptive immunity. This is especially relevant here as it relates to HIV and drugs of abuse.
E. Masliah
I was wondering if you would think that an appropriate addition to your list would be the need for a comprehensive human tissue bank of HIV drug abusers that might serve the community. I think that the greatest challenge, and you pointed this out very well, in terms of the different types of drugs they use, how complex that is—I was wondering if you think that would be useful and even feasible?
A. Nath
As the epidemic is changing, and you know that better than anyone else, a lot of the patients that are coming into the brain banks right now are drug abusers. So I don’t know if you need a separate brain bank for it, but the reality is your existing brain banks probably have a lot of tissue from HIV-infected drug abusers.
E. Masliah
The problem is that in most of the brain banks we have the HIV drug abusers, but we don’t have drug abusers alone, there are no good controls.
Session 1: Glial biology, inflammation and HIV
E. Major
I think there are two areas that we need to look at based on some things that we are doing in the laboratory right now. One is that we need to know much more about the molecular regulation of inflammatory cytokines or chemokines that occur in the context of HIV infection in the brain, whether in drug abusers or not. We know very little about that, and it turns out it’s the astrocyte which is the predominant producer of some of these. I will show you a little bit of data on this. The astrocyte is the stepchild that Avi had talked about. The other is a new target cell within the brain, the progenitor cell, or the stem cell.
To put these issues in context, the first slide shows common elements in infectious disease and neurodegeneration. Inflammatory cells are involved, including macrophages, microglia, and astrocytes, which we have been interested in for a long time. All of these cells produce the inflammatory cytokine CCL2, which used to be called MCP-1, and these are critical components of any type of neurodegenerative diseases. There are common features, as I will show on this slide that is coming up, not just in HIV-associated encephalopathy but some of these more classically defined neurodegenerative diseases, so these are critical components. Now, some years ago when Kathy Conant was in the laboratory, we began to ask that question about CCL2 and whether or not that could be a potential biomarker in cases of HIV-associated encephalopathy, particularly those that go on to frank dementia. The next slide shows published work where we used in situ hybridization to identify the message for CCL2 in HIV-associated dementia cases (Conant et al. 1998); this was also performed at Johns Hopkins (Kelder et al. 1998). It turned out that these particular cells within the brain are astrocytes, and this is a feature associated with multiple sclerosis as well. This is the cell that we use in the laboratory quite extensively to ask some of these detailed questions on molecular regulation of something like CCL2. We had been intrigued from that previous data as to why it is that the astrocyte in the brain is the cell that likes to produce this beta chemokine CCL2. What accounts for that? We are interested in the genetic regulation of molecular factors associated with chemokine production. We use the glial fibrillary acidic protein (GFAP)-positive astrocyte, which we culture directly from the developing human brain at different gestational ages. Although, morphologically, there appear to be multiple cell types in this population, they show a uniform response with respect to HIV infection, other neurotropic viruses, and CCL2 production.
We also have a unique biology that allows us to generate these particular types of cells, initiating differentiation from progenitor cells or stem cells in the brain. One thing we’ve learned comes from looking at another virus, the human polyoma virus JC, which causes progressive multifocal leukoencephalopathy (PML) and still is a substantial neurologic complication in AIDS patients. The astrocytes like to overproduce a certain DNA binding protein that recognizes promoter sequences that are at times glial-specific, and that is the NF-1 family of DNA binding proteins that occur in four different class members. However, the human brain, and glial cells in particular, produces NF-1X in abundance compared with its other class members A, B, and C (Messam et al. 2003). In certain promoter sequences within the brain, particularly in glial cells, there are DNA binding proteins for the NF-1 that are juxtaposed directly next to c-jun or AP-1; this we consider a motif. We call this the “neuroglial box” and we published this in the past (Amemiya et al. 1992). We find this motif in regulation of certain genes from glial cells, for example, in the GFAP promoter and the proteolipid protein promoter.
We then asked whether this particular promoter sequence is responsible for CCL2 production in the astrocyte. Why is it that a glial cell likes to produce beta chemokines? The next slide shows the proximal and distal sequence of the MCP-1 or CCL2 promoters. This is work that was concluded at the time Diane Lawrence was in the laboratory (Lawrence et al. 2006). We found that in the distal end of the human CCL2 promoter you do see this motif, the NF-1 and the AP-1. Could it be that an astrocyte, which produces NF-1 class X that recognizes these promoter sequences and drives the synthesis of the human JC virus, actively participates in CCL2 production here? There are also NFκB sites, an NF-1 site, and an AP-1 site. So we set about trying to dissect out what is the molecular regulation of a beta chemokine either in the presence of HIV or under nonpathological conditions. Part of the data in this paper also showed that as we take the progenitor population of cells and differentiate toward the GFAP-positive glial cell, we increase the synthesis of CCL2 (Lawrence et al. 2006).
Now it turned out that NF-1, although we are still working on this, is not particularly that important as the prime factor for the synthesis of CCL2, NFκB is. The next slide from the cover of an issue of TIBS in 1992 shows the regulation of NFκB within a cell. Signal transduction pathways are of critical importance. That is how everything is initiated, whether it is gp120 and, perhaps Tat, which we know induces CCL2, or it could be phorbol esters, or interleukins. There is a series of cascading events that eventually allows the activation of protein kinase C, which phosphorylates and releases the inhibitor IκB. Then, the p65 and p50 subunits join in this particular type of scary relationship, but it moves into the nucleus of the cell, recognizes the sequences of many different types of promoters including that for CCL2, and initiates synthesis.
So what is it about gp120 or particularly Tat, and are there differences in Tat that we find in individuals with HIV-associated encephalopathy that account for a mechanism that drives NFκB into the nucleus of the cell and selectively allows particularly this beta chemokine CCL2 to be synthesized at a higher rate? It turns out that the astrocyte is the cell in the brain that predominately makes CCL2—that is our hypothesis. The astrocyte plays critically important roles not just in regulation of these types of inflammatory molecules, but it also has a critical role in the birth of different other kinds of cells, particularly neurons (Svendsen 2002; Song et al. 2002). So even in the adult, the astrocyte gives rise to, and actually acts as, a progenitor type of cell and can give rise to other types of cells. Also, since the astrocyte forms a neural–glial synapse (Gallo and Chittajallu 2001), it not only gives rise to novel cells within the brain and responds to injury within the brain, either by producing inflammatory molecules or by producing different cell types, but also plays a role in synaptic connections with neurons.
We were intrigued with the idea that the astrocyte can actually serve as a progenitor type of cell, so we began to look at a series of pediatric AIDS cases. We have collected 62 autopsy cases of pediatric AIDS, clinically very well characterized, with a substantial amount of brain tissue, and we are beginning to screen through these cases to ask the very simple question “Are there HIV-positive, nestin-positive cells within the pediatric brain tissue?” We used in situ hybridization and laser-capture microdissection to evaluate nestin-positive cells in the subventricular zone. We have identified several cases in which we recently identified these types of HIV-infected cells in the hippocampus of pediatric cases, and that is a novel observation. We are beginning to ask whether this particular cell type participates in the pathogenesis of disease.
HIV encephalopathy causes a chronic persistent infection. The molecules associated with this disease are also associated with the more conventional type of neurodegenerative diseases, and HIV-associated encephalopathy is a very good model to study neurodegeneration. We can manipulate the parameters, look at gp120 or Tat, for example, and look for different effects. We can look at different patient populations and ask what occurs during the course of disease—to dissect out questions of pathogenesis, which affect not only this particular type of viral disease but these other diseases as well.
K. Hauser
My laboratory has had a long-standing interest in the role of the opioid system and opiate drug abuse in CNS plasticity. Work published over the past few decades indicates that most cell types in the brain can express μ opioid receptors, which are the principal molecular target for opioid drugs with abuse liability. Subsets of neurons, astroglia, and microglia can express μ opioid receptors.
We have been particularly interested in the response of the CNS to opiates and HIV-1. The CNS is especially vulnerable to the combined effects of substance abuse and HIV-1 infection for reasons that are not completely understood. We have initially taken a reductionist approach to address this problem, which involves examining the direct response of neurons, astroglia, and microglia to opiates and/or HIV-1 in vitro. This strategy allows us to identify the intracellular signaling pathways underlying the response of individual cell types to opiates and HIV-1. Once the response of individual cell types is determined, then the role of intercellular signals between different neural cell types can be assessed. Our inevitable goal is to determine the cell targets and sequelae of intra- and intercellular events by which opiates exacerbate HIV encephalitis (HIVE).
Astroglia appear to be particularly important in mediating opiate-HIV-1 interactions in the CNS. Subpopulations of striatal astrocytes express μ opioid receptors (Stiene-Martin et al. 1998, 2001) and opiates are highly disruptive to astrocytes exposed to HIV-1 proteins. This includes synergistic increases in intracellular calcium, the production of oxyradicals, and in the expression and release of cytokines and chemokines (El-Hage et al. 2005; Hauser et al. 2005).
Using antibody arrays to sample multiple cytokines simultaneously, we found that morphine by itself had minimal effects on cytokine production by astrocytes. By contrast, Tat markedly increased the release of specific cytokines, including interleukins (IL-6, IL-4, and IL-12), and chemokines, including monocyte chemoattractant protein-1 (MCP-1/CCL2), regulated on activation, normal T cell expressed and secreted (RANTES/CCL5), and MCP-5 (CCL12). Importantly, however, when morphine and Tat were combined, there were synergistic increases in the release of MCP-1 and RANTES, in particular (El-Hage et al. 2005). Based on this finding, we hypothesized that astrocytes, through their ability to signal to other cell types in the brain, may be important intermediates for opiate drug–HIV-1 interactions. We took this concept further and found that intrastriatally injected Tat caused a gradient of inflammation (El-Hage et al. 2006b), which was, in part, defined by MCP-1 immunoreactivity (El-Hage et al. 2006a). At 300 ± 100 μm from the injection site, there were increases both in GFAP immunopositive astrocytes, as well as astrocytes that coexpressed MCP-1 following Tat or morphine exposure. By contrast, these changes were not evident farther (600 ± 100 μm) from the site of Tat injection. When morphine and Tat were combined, they caused additive increases in the proportion of MCP-1 immunoreactive astrocytes. The effects of morphine were prevented by concurrently administering the broad-acting opioid antagonist, naltrexone. Quite interestingly, the damage caused by inserting a sterile syringe into the striatum seems to be sufficient to up-regulate the expression of μ opioid receptors and MCP-1 immunoreactivity in astrocytes (El-Hage et al. 2006a, b). This suggests that astrocytes innately respond to a variety of insults by releasing chemokines and by up-regulating μ opioid receptors.
We further assessed whether morphine- and Tat-induced increases in astroglial-derived chemokines are accompanied by corresponding increases in macrophages/microglial activation (El-Hage et al. 2006b). The results showed that combined morphine and Tat caused marked increases in macrophages at 300 ± 100 μm, but not 600 ± 100 μm, from the site of Tat injection. Detailed studies are examining the temporal patterns of macrophage recruitment/microglial activation and the extent to which this coincides with neuronal injury.
To assess the role of MCP-1 in opiate and HIV-1 Tat-evoked glial activation, the effects of morphine and Tat were assessed in mice lacking CCR2, which is the cognate receptor for MCP-1. In CCR2 null mice, there was a marked reduction both in astrogliosis and macrophage/microglial activation in response to Tat or combined morphine plus Tat exposure compared to wild-type mice (El-Hage et al. 2006a). The results suggest that MCP-1, acting via its cognate receptor CCR2, contributes to inflammation caused by morphine and Tat. The role of other chemokines such as RANTES additionally needs to be assessed.
A hypothetical model showing how opioids act through astroglial intermediaries to exacerbate HIVE is presented (Fig. 4). HIV-1 normally disrupts astroglial function and causes the release of specific cytokines and chemokines. In HIV-1-infected individuals, opiates exacerbate the inflammatory effects of viral products in the subpopulation of astroglia that express μ opioid receptors. Chemokines from astrocytes recruit monocyte/macrophages from the periphery into the CNS and activate microglia. Activated macrophages/microglia, for example, release excessive amounts of glutamate, quinolinic acid, nitric oxide, reactive oxygen species (ROS), and arachidonic acid, which can be neurotoxic. In addition, astroglial-derived chemokines are likely to increase the recruitment of HIV-1-infected peripheral leukocytes into the CNS and increase the number of resident glia that become infected. As noted, neurons are not infected by HIV-1, but are injured by exposure to viral proteins and inflammatory agents released from infected glia. Morphine exaggerates the inherent response of astrocytes to HIV-1 proteins. Losses in calcium homeostasis and increased oxidative stress are likely to impair astrocyte function, which may limit their ability to buffer extracellular glutamate and potassium and further promote neuronal injury.
Diagram illustrating how opiate-induced changes in HIV-1 exposed astrocytes contribute to HIV-1 encephalitis. Opiates synergistically destabilize ion homeostasis and increase the release of proinflammatory cytokines and chemokines by HIV-1 protein (gp120 or Tat) exposed astrocytes. Astroglia likely modify the intrinsic response of neurons and macrophages/microglia, which also express MORs, to opiates and HIV-1. Solid arrows indicate intercellular events signaled by astroglia, whereas the dashed arrows denote intercellular signals originating from macrophages/microglia. BBB, blood–brain barrier; IL, interleukins; MCP-1/CCL2, monocyte chemoattractant protein; MOR, mu opioid receptor; RANTES/CCL5, regulated on activation, normal T cell expressed and secreted; additional descriptions are provided in the text (modified from Hauser et al. 2005).
Regarding future questions, the direct effects of opioids and HIV-1 need to be better understood in neurons and macrophages/microglia. Moreover, the collective tissue response is likely to differ greatly from the response of individual CNS cell types. For example, morphine suppresses motility and phagocytosis in isolated microglia. By contrast, if microglia are cocultured with astrocytes, morphine markedly increases microglial activity (El-Hage et al. 2006b). Thus, the response of microglia to opiates is contextual and can be modified by factors released from astrocytes. Understanding the primary targets of opiate–HIV-1 interactions in the CNS and subsequent chain of events contributing to HIVE is important for designing therapeutic interventions in HIV-infected substance abusers. Moreover, because opiate drugs act by mimicking endogenous opioid peptides, which are normally present in the CNS, understanding how opioids contribute to disease progression is also likely to be beneficial in HIV-1-infected individuals who are not substance abusers.
Another important question is, what are the appropriate model systems to examine NeuroAIDS and drug addiction? This is a complex issue and perhaps no single model is ideal; each has inherent strengths and weaknesses. For example, the intrastriatal Tat injections described herein are advantageous for examining chemotaxis toward a focal site of inflammation and the role of MCP-1/CCR2 in inflammation; however, this strategy is less desirable for measuring biochemical changes or dendritic/synaptic losses because of the nonuniform response of cells within the Tat gradient. We are using an inducible Tat-expressing transgenic mouse in collaboration with Dr. Avindra Nath and SCID mice inoculated with HIV-1-infected human monocytes in collaboration with Dr. William Tyor as alternative models for HIVE. Modeling human addiction in animal models is a challenge and requires careful consideration of the complex patterns of drug use in addicts and sensitivity to pharmacological differences between species.
J. Berman
I chose to talk to you about ideas and some new data that we have rather than the published data, but I am going to give you just a 1-min summary of the published data so you can understand the context in which we are asking the questions that are to follow. Our laboratory has an in vitro tissue culture of the human blood–brain barrier. It has barrier properties, and expresses proteins similar to the human blood–brain barrier. We recently showed in Journal of Neuroscience that HIV infection and CCL2 are critical for exuberant transmigration of HIV-infected cells across this model, and that this causes disruption of tight junction proteins (Eugenin et al. 2006b). We are analyzing the mechanisms by which these two key factors, infection and CCL2, mediate a breech of the blood–brain barrier in CNS infection (D’Aversa et al. 2004, 2005; Eugenin et al. 2005, 2006a; King et al. 2006; Buckner et al. 2006).
In that context, we started to introduce cocaine, dopamine, etc., because our patient population in the Bronx has a large percentage of substance abusers. What is important to say is that I stand on the shoulders of two significant giants in this clinical component of our research, Drs. Ellie Schoenbaum and Robert Klein, who are spearheading patient cohort studies of over a thousand individuals in the Bronx, including men and women who are HIV-infected or uninfected substance abusers. It is critical to address the questions of how substance abuse and HIV in concert affect CNS function. Drug abuse is obviously a major factor in the spread of HIV, and the incidence of NeuroAIDS appears to be somewhat higher or accelerated among drug abusers. I do want to point out that Avi Nath talked about the indigent population that is underinsured, and that is why pharmaceutical companies may not be willing to address some of the important issues. I think that is certainly true, but we need to also remember that it is not just the poor population that is infected with HIV. Many “affluent” communities or comfortable communities have substance abusers, so this a very general epidemic or pandemic.
Drugs of abuse act through activation of specific receptors on many different cell types and cause alterations in synaptic plasticity as well as alter viability of neurons. Cocaine is our interest, because it is a major drug used in the Bronx; and it acts by interaction with dopaminergic, serotonergic, and norepinephrine neurons, and especially their transporters. Mechanisms by which drug abuse potentiates NeuroAIDS are not well known. The question, of course, is how does HIV alter CNS and immune cells and vice versa in the context of these neurotransmitters and substances of abuse? This is unanswered and additional approaches as Avi Nath described are necessary. I will discuss some of those at the end.
Drug abuse will cause dysregulation of normal CNS functions. Our particular focus is dopamine, serotonin, and norepinephrine, and associated systems. HIV can come into this context in many different places, and there are alterations in both the periphery and CNS, especially neural and glial cells that control homeostasis of neurotransmitters and facilitate the inflammation that enhances HIV infection of the CNS. The function of the glial cells, as Gene Major was talking about, is to control the CNS environment for neuronal function, such as by recycling of extracellular toxins. These cells also amplify the inflammatory response by elaborating cytokines and chemokines that recruit circulating leukocytes, as well as compromise the blood–brain barrier and neuronal integrity.
What is known in NeuroAIDS is that HIV infection clearly alters blood–brain barrier integrity and neuronal survival, enhances transmigration of leukocytes into the brain, and enhances expression of inflammatory mediators. Now, add neurotransmitters to the backdrop of all of these factors, and there are many important questions to ask. So, again, we have cocaine and dopamine, serotonin, noradrenaline dysregulation. What is the source of these transmitters? Is it the adrenal glands? Is it HIV-infected leukocytes? Is it from the CNS or other cell types? I am going to put forth an unconventional hypothesis to perhaps address some of these questions. The potential consequences of dysregulation of these neurotransmitters are very broad: there is blood–brain barrier disruption, changes in HIV replication, neuronal or glial alterations, and certainly inflammation.
Important questions are, “What is the time course of action of these neurotransmitters during the course of AIDS and NeuroAIDS?” We need to use alternative approaches such as imaging, electrophysiology, neurochemical and biochemical techniques, 2D gels, etc., to identify important mediators of this process. Do these neurotransmitters change the patterns of inflammation? Does inflammation change the patterns of these neurotransmitters?
Our hypothesis is based on very little data, so we are unfettered by fact. The hypothesis is that cocaine dysregulation of neurotransmitter homeostasis in the periphery contributes to the early phases of NeuroAIDS by altering immune cell activation and blood–brain barrier function, resulting in accelerated neuroinflammation. Now that’s not to say that cocaine doesn’t act in the CNS; obviously, it does. However, we propose that some of the earlier changes that we are seeing are a result of increased dopamine and serotonin in the periphery, and that later on the accumulation of these neurotransmitters in the CNS helps to enhance neuronal dysfunction. So that at later stages these neurotransmitters continue to act on CNS cells, further enhancing inflammation and cell damage.
This hypothesis is based on only two pieces of data, and the next slide shows one of them. This is from sera collected from our patient cohort. These patients have been extensively evaluated for substance abuse, alcohol abuse, and HAART, and they have been subjected to both minimental and recent neuropsychological evaluations. The first thing I will tell you is that HIV infection, substance abuse, or the combination thereof, is enough to cause significant levels of circulating dopamine. In addressing one of the issues that was brought up earlier, the control population is twofold. One control is people from my laboratory, and the other control is within the population group and the only thing that control means here is that they are not HIV-infected. They are substance abusers, they are alcohol abusers, etc., they are just “not” HIV-infected. So here is the control population, and over here is the control population that has been deemed neurocognitively impaired. These other bars show the populations that are HIV-infected or HIV-infected with neurological impairment. As you can see, these are enzyme-linked immunosorbent assays (ELISAs) on the sera for dopamine and serotonin. As soon as you introduce cocaine, HIV, or HIV with dementia, there are elevated levels of serotonin and dopamine. I do not think these levels are being spilled out from the brain because I don’t think that these people, for the most part, are malfunctioning except for the group with neurocognitive impairment. I’m going to suggest that it is coming from the periphery, and that this dopamine is going to act on the HIV-infected cells, alter their chemotaxis properties, enhance their ability to enter the brain, and also disrupt the blood–brain barrier. Dopamine decreases ZO-1, which is a tight junction protein in human microvascular endothelial cells. We will treat our blood–brain barrier with dopamine to determine if there is an increase in cell permeability. We will examine whether there are dopamine receptors expressed on our neuronal blood–brain barrier, and we are beginning to do 2D gel analysis of dopamine-treated brain endothelial cells and astrocytes. We will perform microarrays on HIV-infected peripheral blood mononuclear cells (PBMCs) treated with dopamine and also treated with dopamine plus CCL2, because we know that is a critical mediator of HIV entry into the brain.
So there are many unsolved mysteries that we need to discuss. How does HIV in the periphery and/or CNS cells alter glial cell activation or peripheral cells in the context of dysregulation of neurotransmitters? Is this a direct mechanism mediated by the virus? I don’t think it is. What is the time course of NeuroAIDS and synaptic impairment in the context of drug abuse? What are the major cells and tissues affected early on that result later in neuronal dysfunction, and is this a pathway for therapeutic intervention? What is the sequence of events? As noted earlier, we need a series of both large and small animal models to address these issues. To improve our approach to these studies, we need techniques such as electrophysiology, live and general imaging, and neurochemical analyses. What I will say to the NIDA group in terms of funding initiatives, one of the problems affecting a lot of these studies is that we are working with live virus and we need to see their dynamic interactions, not their fixed interactions with different cell types, and it becomes difficult to use imaging equipment that is not HIV dedicated. As this equipment is expensive, perhaps there could be a shared instrument initiative or something that would fund the purchasing of the equipment that is needed to address a lot of these technologies that would help move us forward. Live intravital microscopy, etc., just can’t be done in an HIV-infected context in most institutions.
M. Carson
I’m not going to mention either drugs or HIV, but I’m going to be talking about microglia, and I want to put onto the table some of the complexities that need to be considered when you are studying drug interactions or viral insults. There are times when people come to me and say, “When do microglia start doing all those bad things? They are clearly off in a normal brain, and then when they are turned on they are basically designed to melt the brain down.” That is sort of a naïve first look of the literature. One thing I want to share with you is that although we are frequently set up to think about microglia as pathogenic, nonadaptive, or useless cells in the brain, microglia are highly interactive with their environment. They are really great biosensors, and by integrating so much from their environment it’s not surprising that they are going to be very heterogeneous in their effector function phenotypes. They are adapted to specific brain regions, to specific neuronal activities, and are very sensitive to changes in these. We really have to be cognizant of this. Also, because these cells are so interactive with their environment, plastic in their functions, and heterogeneous in their original starting stage, we have to be careful of the models we use, because they frequently drive the results that we obtain (Carson et al. 2006).
Here, I’m showing a microglia in a healthy mouse nervous system. When you are using lectin, you can really see all the processes. If you use CD11b Mac 1 staining, you cut the cells at the elbow, and you don’t always realize how much the processes extend out. One of the things to notice is that they really like to cozy up to everything in their environment, touching every cell there. Microglial cells are incredibly interactive.
The problem I want to discuss here is that when we are looking at microglial function—whether we are thinking of it in terms of drug use, AIDS, or interactions between the two—we have this basic problem: “What model of microglial function should we study, and then what should we assay?” Microglia are called the tissue macrophage of the brain, therefore we are going to look to the peripheral immune literature, and we are going to look at those great assays that have been developed, and we are going to look for really strong cytokine responses and adaptive immune responses and their ability to regulate T cells. That is great, but is that what they are doing or is that just what we have developed assays to look for?
This is the kind of thing where you look at the literature and see microglia from two different viewpoints. One is that they are essentially off in the healthy nervous system and they are kept off by essential interactions with neurons and astrocytes, CD200, and various other molecules expressed by neurons we do know actively down-regulates them. We do know that microglia express receptors for various neurotransmitters (such as the work of Jonathan Sedgwick, Helmut Kettenmann), and that interactions with those neurotransmitter receptors on microglia tend to down-regulate the responses to subsequent encounters with pathogens, such as lipopolysaccharide (LPS) and viruses. So the cells are sensing what neurons are doing. It has been in the literature that they are kept actively off by the environment and then somehow the pathogenic insult comes in and flips them over to a totally wild activated state.
There is also an idea of a continuum of microglial activation. You are activating in order to kill pathogens—that is their only function that we can assay, so that must be what they do. They make toxic molecules and become killer cells, and we are just going to balance having to kill our pathogen versus tolerating CNS damage.
This is clearly the first cell that can almost always monitor reactive changes in CNS homeostasis. It is important to realize when you look at microglial activation in the CNS, whether you hit your head on the kitchen cabinet or induce a change in LTP in your laboratory, or any other changes in neuronal activity that are not huge pathogens, your microglia become activated. You can see changes in microglial gene expression within minutes of changing neuronal activity, and yet, most of us aren’t having total brain meltdown. So, they are clearly able to get activated and do things and have interactions that are adaptive. It is a very important point that microglial activation by itself is not maladaptive, but because their functions are very plastic, they are really dependent on the signals that they get from their environment. If you have done things that really are changing neuronal activity or causing neuronal dysfunction, and this can be drug abuse, other kinds of pathogens, or changes in astrocytes, you are going to have severe changes in microglial activation and severe changes in how microglial function is regulated. Clearly, in the case of HIV and other situations where we have a primary dysfunction in microglia due to either genetics or pathogen infection, they are going to mis-summate this information and develop inappropriate responses.
It is important to make the distinction of where the dysfunction is—at the neurons, at the astrocytes, or within the microglia—and what the combination is. One of our approaches to understanding the cells has been a molecular one. We can look at gene expression in microglia, such as riboprobes for specific molecules. We use lectin, which will stain microglia and macrophages in blood vessels, and we are then able to localize gene expression. Using that approach for many different models, we can compare gene expression of a whole slew of molecules that are expressed in microglia. We were routinely doing this with a panel of about 50 molecules, and we have a few other viral models we use as well. When we take things such as facial axotomy, Wallerian degeneration, rapid acute resolving inflammation, LPS/IFN-gamma intercerebral injection, amyloid pathogenesis in transgenic models, experimental allergic encephalomyelitis (EAE), or toxin-mediated demyelation and remyelination, you don’t see one pattern of microglial action, on or off. You don’t even see a graded pattern. What you see, even looking in this limited panel of five molecules, is a specific, pathology-dependent pattern of gene expression. We even see spatial patterns of differences, between two places where we have demyelination. Microglia really are summing their environment. Their responses are so specific to what they have seen and what we can also show is that they carry their history with them. So, if they have had one encounter they are now in one state, so it is not a continuum. Then you ask them to respond to something else, and then they will go into another state. That is different than if they were over here and then had that same encounter. These are very simplistic ideas, but now we are able to actually have molecular tools to pin that down.
There are several molecules that might be differentially expressed between microglia and macrophages. Something we always bring up in our laboratory is that everybody’s slide says microglia/macrophages because you can’t in histology tell the difference between them. The problem is they aren’t identical so they can have different responses, and the other thing is microglia can be different throughout different brain regions. Part of the reason we have problems understanding what microglia and macrophages do is that frequently we use cultured models, and I am one who does that. When we have done profiles, we have molecules that look like they are microglial enriched, not really expressed in macrophages, but then we look in vivo and they are not expressed by microglia. Cultured microglia do have functions that don’t even exist in vivo, so it is hard to know what we are studying. I am not casting stones because if you read our work we have used a lot of cultured microglia because we have to, but it is something we have to be cognizant of, why some of the times our in vitro work is not predictive of our in vivo efforts.
To show you there is a difference between microglia and macrophages, we can take John Sedgwick’s method of segregating microglia, or activated microglia, from CNS-infiltrating macrophages based on their relative levels of CD45 expression. We can take various models; here, I am just talking about one where we do an intercerebral injection of LPS/interferon gamma, 50,000-fold lower levels than what one does to induce neurodegeneration for Parkinson’s. We can assay, do these cells have different functions? If you put microglia into cocultures with hippocampal neurons, both cell types are quite happy, but that is clearly not the case with macrophages. So that tells you in a nutshell that when we are looking at microglia and macrophages, even though we can’t always tell the difference histologically, even though they are in the same environment, we can’t say that they are doing the same things.
So the perception, the rules, and the abilities of microglia can be the function of the model being used, we tend to find what we look for. Microglia activation is complicated and this is very obvious, and this is because they are heterogeneous cells and we are often confounding microglia with macrophages. I would like to suggest that activation states might not be strictly beneficial or detrimental. They could be appropriate versus inappropriate. For instance, some of the things we see in amyloid pathogenesis, the microglia are being pushed to do neuroprotective antigen presentation, but they are down-regulating their ability to phagocytose, and in an amyloid situation they should be phagocytic, but they aren’t actually doing something bad. That means we need to be paying attention when we are thinking about therapies not just being totally immune suppressive to everything, but really trying to understand the functions of these cells.
Discussion following session 1
H. Fox
One thing you didn’t mention about microglia is their motile states. You showed all the pretty pictures, but as you know studies with mice that express GFP in microglia made by Dan Littman’s laboratory showed that those processes are actually dynamic—they are swimming around at all times. How can we study this? What is the challenge as far as drugs of abuse and infectious disease, if not HIV?
M. Carson
I think that actually the big issue is with imaging. It is incredible, that is a 5-μm slice up there and it looks very static, but there is this gorgeous work published last year in Science that has some very nice two-photon imaging that I would really refer people to look at. It shows you the thousands of contacts the microglia are doing, which astrocytes are also doing. So, you have this great complexity of the glia and the microglia constantly sampling the entire environment, and being very interactive. It is very interesting, if you look at recently presented work by Helmut Kettenmann, that neuropeptides alter the sampling rates of these cells, and also change their motility in response to pathogenic stimuli. So I think this is a very important point that while dopamine and serotonin, what I call the happy transmitters, somewhat down-modulate their proliferative and migratory capacities, glutamate totally amps their proliferative and hyperresponsiveness. One of the things I always say when we look at our mixed glial cultures or microglia in isolation, is what am I studying there? I’m studying a cell that is either in the presence of proliferating glia, so that must be a glioblastoma, or I am studying cells in isolation. Their responses are very different.
J.S. Hong
Certainly a very interesting point in terms of the heterogeneity of the microglia, the question is do you think they are different to begin with, or do they represent a different stage of activation, therefore, the phenotype appears very, very different?
M. Carson
That is a complex question, and the data are still coming out. I would favor the interpretation that early in development the microglia are more homogeneous and our gene expression profiling does tend to support that fact. When we look at a large panel of molecules, as that beautiful fountain head is in there, and as you see in a population within the embryonic and postnatal environment, the gene expression is much more uniform. Not entirely, but much more. In a mouse, at day 11 there is a lot of synaptogenesis, eyes opening, a lot of myelination is completing. We start really seeing overt heterogeneity appearing. I would favor that the environment drives the phenotype, however, once you have the heterogeneity, they seem to be developing or differentiating differently in different parts of the brain region, and then when they hit a pathogenic response they respond differently. If you come back with a second pathogen later, you have even different responses. So they carry their history with them somewhat, and that is also a general feature of the immune system, so I don’t think it is totally novel. So I think of it as sphere activation. Microglia start off; however, microglia from another brain region may respond differently. This could be a significant issue if one considers the migratory capacity of microglia within the CNS.
J. O’Callaghan
Am I hearing that it is pathogen-related phenotypes with the different types of microglia, or am I hearing the need for more markers, if you will, of subclasses of microglia?
M. Carson
Both. I would say it is environment-driven.
E. Masliah
I am quite fascinated with the work of Dr. Major on neurogenesis and HIV infection. I was wondering if you could comment on whether you have done similar studies with drugs of abuse. Have you looked if methamphetamine or morphine somehow quiets neurogenesis? What happens in that regard?
E. Major
No, we haven’t initiated that kind of work. But with the ability to identify progenitor cells both in culture systems, as well as in tissue, I think all the comments we’ve just heard could be applicable to a variety of cell types that we find in the brain. The dynamic nature of cells compared to the fixed nature of cells in pathologic tissue, or compared to the biology of the models systems, is applicable to microglia cells, astroglial cells, and/or neurons. Our laboratories generally tend to look more at molecular regulation. An area that needs much more attention and much more of an understanding is how the brain responds to injury; repair and regeneration involving the stem and the progenitor cells. They can be affected through infection or through drugs or through injury. I think we are at a point now where we have to begin to look at some of these critical questions of how the brain responds to injury.
A. Nath
The elevated dopamine levels were quite interesting in those HIV patients. The major source of dopamine in blood is actually platelets and platelet dysfunction is known to occur in HIV-infected individuals, so I was wondering what your thoughts on that are, and if you think platelets may be the source of dopamine.
J. Berman
We know that platelet progenitors, megakaryocytes, are infected with HIV and certainly could be pouring out the dopamine. The adrenal glands, perhaps also, we are looking at that. HIV-infected leukocyte cells also produce a lot of dopamine, but not enough to account for those levels. I certainly don’t think there is a compromised blood–brain barrier in most of these patients, but rather that it’s coming from the periphery—it could be the result of several source contributing; the fact that dopamine is present in such huge quantities could have tremendous impact both on the ability of the affected cells to respond to chemoattractants, and also to the integrity of the blood–brain barrier.
C. Power
Just to echo the conversation this morning about the heterogeneity of microglia, it’s the same as astrocytes and perhaps even more so. We have very limited tools in terms of markers for astrocytes. Do you have any comments or do you know of new libraries of antibodies available to characterize astrocytes?
E. Major
As far as I know, there are no new libraries for these types of cells and we’ve had an interest in taking the culture models that we have and separating them out in different morphologic types if that’s possible. In both the work we’ve done with the human polyoma virus JC, as well as HIV in these culture models, we find that regardless of the characterization of the astrocyte population in culture, the response to infection for either of those viruses is similar. GFAP-positive cells from an 8-week gestational human developing brain compared with an 18-week gestational age developing brain have similar responses to JC virus (lytic or chronic infection) and to HIV (a nonproductive persistent infection).
J.S. Hong
Cytokines in the blood are very effective, very powerful in altering the barrier of the brain. Have you looked to see if cytokine levels, especially TNF-α, are very high in some infected people? Could this be a synergistic effect between dopamine and some of the cytokines?
J. Berman
Absolutely, and that’s a major question that we’re interested in, particularly dopamine and CCL2. There are data from our laboratory and from Dr. Joel Pachter’s laboratory and others that CCL2 alters the integrity of the blood–brain barrier. It is subtle, but actin fibers, etc., do change. I think there’s a lot of cooperation among dopamine and other inflammatory mediators. Treatment of our blood–brain barrier model with individual factors does not make a dramatic difference, but when combinations of factors are used, there is often a dramatic change in permeability.
W. Royal
I was also very intrigued by your findings, and they made me think of work that Dr. Steve Maier and his colleagues have done at the University of Colorado. Neurally disconnecting the CNS from the peripheral immune system. This resulted in the blockage of the effects of systemically administered IL-1-beta on changes in CSF catecholamine levels. Are there any subjects in your cohort who might have undergone surgical or “chemical” splenectomy, and, therefore, interruption of such vagal pathways?
J. Berman
There is a group of individuals who are alcohol-dependent and we can get those data. The beauty of these cohorts is that there are 1,000 patients so that the statistical nightmare that Avi referred to is still there, but we can reliably evaluate the data. We have not seen a difference specifically, but we did not subdivide the groups for alcohol use.
K. Hauser
Back to the heterogeneity issue, there are huge differences in pharmacological receptors among astrocytes. For example, we find that opioid receptor expression is highly plastic in astrocytes. Subsets of astrocytes can express any combination of μ, δ, and κ opioid receptors, whereas many astrocytes fail to express opioid receptors entirely (Stiene-Martin et al. 1998). The events regulating opioid receptor diversity among astrocytes appear to be complex. Opioid receptor expression is developmentally regulated and differs among astrocytes from different brain regions. Moreover, δ opioid receptors are regulated in a cell cycle-dependent manner, suggesting that during ontogeny a single astrocyte may change its phenotype. How astroglial and microglial heterogeneity (mentioned earlier) is defined by or contributes to local inflammation or drug interactions is uncertain, but likely to be important.
Session 2: Pharmacology, neurotoxicity and neuroprotection
J.S. Hong
I will present a general review over our current view of the role of inflammation in neurodegenerative diseases, the development of an inflammation-based model to study the mechanisms of inflammation, and finally, the development of potentially novel neuroprotective drugs. My talk will focus on the two cell types, astroglia and microglia. These glial cells are important players in neurodegenerative diseases and I will emphasize why they are prime targets for therapy. Astroglia, which are a good source of neurotrophins, are responsible for the neuronal survival. Overactivated microglia may trigger uncontrolled inflammation and consequently damage neurons (Block and Hong 2005). Thus, microglia are prime targets for anti-inflammation therapy.
Initially, we used LPS to develop in vitro and in vivo models to induce neurodegeneration and mainly used Parkinson’s disease as a disease model. However, LPS can be applied to different kinds of disease models. We have used LPS to activate microglia to produce a range of proinflammatory factors, which in turn, killed neurons (Fig. 5). The most widely used model to study Parkinson’s disease is 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is believed to induce neurodegeneration directly. We have previously observed that the presence of microglia enhances MPTP-induced neurotoxicity. So what could be the role of microglia? It turns out that reactive microglia plays a very important role in enhancing the MPTP-induced neurotoxicity. It doesn’t matter how the neurons are killed or damaged by LPS or MPTP; the damaged neurons send signals to activate microglia to clean up, by phagocytosis, the debris from damaged neurons. However, in the process of the activation, microglia tend to secrete more proinflammatory factors, which lead to further neuronal death and in turn causes more reactive microglia. The vicious cycle continues and produces more neuronal death. We use this model to explain why most of the neurodegenerative diseases are progressive in nature, including HIV dementia. Most of the patients need at least a few years, or up to 10 years to develop symptoms. Our data indicate that microglia play a key role in the progression of these diseases (Fig. 5).
Role of microglia in toxin-induced neurotoxicity. LPS is an indirect neurotoxin that activates microglia to secrete proinflammatory factors that damage on dopaminergic (DA) neurons. In contrast, MPTP directly and selectively damages DA neurons, although the presence of microglia enhances MPTP-induced toxicity. A wide range of toxins, including pesticides and endogenous toxic proteins, produce neurotoxicity in similar patterns; high concentrations act directly, whereas low concentrations mainly target microglia. In addition, signals from damaged neurons activate microglia, leading to microgliosis. Activation of microglia, secretion of proinflammatory factors, the death of neurons, and the reactive microgliosis, form a vicious cycle. It is likely that this vicious cycle is critical for the self-propelling force underlying the progressive nature of neurodegeneration.
One point I would like to mention here is that between two extremes, LPS and MPTP, we studied a variety of toxins including rotenone, paraquat, and a number of misfolded and aggregated proteins, including β-amyloid, α-synuclein, and to some degree HIV-1 gp120 (Gao et al. 2002). There is a very consistent pattern in exerting their neurotoxicity among this group of toxins. These toxins have already been reported to directly damage neurons in high concentrations. Our laboratory found that in the presence of microglia, only a tenth or even less of the toxin concentrations are sufficient to produce neurodegeneration. The reason for the enhanced neurotoxicity is due to the activation of microglia by these toxins, similar to the way LPS produces neurotoxicity by the activation of microglia (Kim et al. 2000).
People often ask whether inflammation is a major cause, or a consequence, of the disease process. The answer could be both. According to our model of reactive microgliosis, it doesn’t matter how microglia are activated—directly by LPS or indirectly by damaged or dying neurons—they assist in accelerating neuronal damage through the self-propelling cycle. This vicious cycle not only provides a molecular model to further understand the progressive nature of neurodegenerative diseases, but also serves as a useful target for therapeutic interventions (Block and Hong 2005). The strategy is to halt or slow down the cycle by preventing the overactivation of microglia. Microglial overactivation triggers inflammation and thus, targeting microglia is a useful strategy for anti-inflammation. Conventional anti-inflammatory drugs such as aspirin, cyclooxygenase-2 (COX-2) inhibitors, or receptor antagonists for cytokines target only one or two of the proinflammatory factors by inhibiting the COX-2 enzyme, which inhibits the production of free radicals, cytokines or prostaglandins. These anti-inflammatory drugs are not effective due to the following reason: when microglia or other immune cells are overactivated, they secrete a wide spectrum of proinflammatory factors; therefore, by targeting only one or two factors, these conventional drugs do not completely prevent the onset of inflammation. High dosages are needed and the resulting side effects can be a serious problem after long-term usage.
We employed a different strategy by designing drugs that will regulate microglial activity and prevent overinflammation (Qin et al. 2005). We discovered some old drugs that are structurally related to morphine. These drugs, called morphinans, include naltrexone, dextromethorphan, and even endorphin-related peptides, and are potent anti-inflammatory and neuroprotective agents both in in vitro and in vivo studies (Zhang et al. 2005).
The next slide shows why these drugs are effective in preventing the overactivation of microglia. These novel anti-inflammatory agents do not target downstream products but rather affect a membrane-bound enzyme called NAPDH oxidase. This enzyme, which is expressed by most phagocytes, is a major superoxide-producing enzyme. We hypothesized that if we can control its activity to prevent the overproduction of superoxide, then we can achieve the anti-inflammation goal (Block et al. 2006). We have learned that superoxide generated by NAPDH oxidase has dual functions: one is to combine with nitric oxide to form toxic peroxynitrite metabolite, which exerts direct damage on neurons; the second way is its diffusion back to the microglia to regulate proinflammatory expression, such as TNF-α, interleukin-1 beta (IL-1β), and a whole host of chemokines. Since NAPDH oxidase plays such an important role in regulating inflammation, targeting this enzyme turns out to be a very useful strategy for developing potent anti-inflammatory drugs (Liu et al. 2002; Qin et al. 2005).
I would like now to shift my discussion to astroglia. From the neuroprotection point of view, astroglia are major sources of many neurotrophic factors, including glial cell-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF). We discovered a number of compounds that have an anti-inflammatory effect, but also can stimulate astroglia to secrete a series of neurotrophic factors to produce neuroprotection. For example, 3-hydroxy-morphinan, a natural metabolite of dextromethorphan, is much more potent than its parent compound in neuroprotection because of its anti-inflammatory effect on microglia as well as its ability to stimulate neurotrophic factor secretion from astroglia (Zhang et al. 2005). Therefore, drugs with multiple sites of action will be the choice for treating neurodegenerative diseases.
J. O’Callaghan
Most of the findings I will present today are attributed to in vivo responses of microglia. I will present data showing that drugs of abuse can lead to the induction of potentially adverse proinflammatory responses, as has already been indicated by other presenters. Of course, the conclusions we reach based on our research findings are only as good as the models we use. Therefore, my laboratory has dedicated a great deal of effort to the development of neurotoxicity models using known dopaminergic neurotoxicants as positive controls. Much of our efforts have been focused on MPTP, the known human and mouse dopaminergic neurotoxin. In our MPTP model, we administer a single low dosage (12.5 mg/kg, s.c.) to the C57Bl6/J mouse to cause an approximately 50% loss in dopamine nerve terminals as reflected by the observed decrease in striatal dopamine and tyrosine hydroxylase. Recently, we developed a single dose methamphetamine model (20 mg/kg, s.c.) to achieve the same level of dopaminergic terminal loss seen following MPTP. We used these single-dose models to establish the time course of dopaminergic nerve terminal degeneration relative to the attendant microglial and astroglial activation responses seen as a consequence of the damage caused by these agents (e.g., O’Callaghan and Sriram 2005; Sriram et al. 2006b). We found that the expression of a variety of proinflammatory cytokines and chemokines was associated with the earliest stages of damage caused by these dopaminergic neurotoxicants.
Using real-time polymerase chain reaction (qRT-PCR) quantification of mRNA for IL-6, CCL2 and TNF-α, we found large increases in these cytokines/chemokines during the earliest stages of dopaminergic damage due to MPTP or METH (Fig. 6A). The rapid time course of these effects—effects largely terminated at 24 h postdosing—parallel the activation of microglia, as assessed by lectin staining (e.g., see Streit et al. 1999). If the expression of these proinflammatory mediators played a role in the dopaminergic neurotoxicity caused by MPTP or METH, we reasoned that suppression of microglial activation might serve as a means to modify neurotoxic outcome. We pretreated mice with large doses of the tetracycline antibiotic, minocycline, a known inhibitor of microglial activation in other models of dopaminergic neurotoxicity (Wu et al. 2002). Indeed, in both the MPTP and METH models of dopaminergic neurotoxicity, minocycline at least partially suppressed the activation of IL-6, CCL2, and TNF-α (Fig. 6B). When we subsequently examined the effects of minocycline pretreatment on MPTP- and METH-induced neurotoxicity, we failed to see neuroprotection (as assessed by change in TH) or any attenuation of damage-related astroglial activation (as assessed by analysis of GFAP) (Fig. 6C). These findings were indicative either of a lack of involvement of cytokines/chemokines in MPTP and METH neurotoxicity or of a requirement for a more complete antagonism of the expression of a given cytokine/chemokine.
Microglial associated cytokines and chemokines: induction by neurotoxic regimens of MPTP and METH and effects of minocycline and genetic deletions. (A) MPTP and METH induce a rapid, large, and time-dependent increase in mRNA for the microglial associated cytokines and chemokines, IL-6, CCL2, and TNF-α. (B) Minocycline (100 mg/kg) attenuates the expression of these cytokines/chemokines in MPTP and METH-treated mice. (C) Despite attenuation of factors associated with microglial expression, indices of MPTP and METH neurotoxicity remain unaffected by minocycline. (D) Genetic ablation of TNF receptors, but not of IL-6 or CCL2, confers neuroprotection against MPTP-induced neurotoxicity.
To resolve these possibilities, we turned to a genetic approach to the problem. Specifically, we administered MPTP to mice lacking IL-6, CCL2, or both TNF-α receptors. Using both TH and GFAP levels as indices of neurotoxicity, it is evident that the TNF-α receptor double “knockout” mice were protected against the neurotoxic effects of MPTP (Fig. 6D). Knocking out IL-6 and CCL2 did not confer a similar neuroprotection (Fig. 6D). Similar findings were observed in the knockout mice treated with METH (data not shown). Together, these data implicate TNF-α as a participant in the dopaminergic neurotoxicity caused by MPTP and METH in the mouse. Our data suggest a potential role for TNF-α in dopaminergic neurodegeneration underlying the earliest stages of Parkinson’s disease. It is important, however, not to overgeneralize the role of this proinflammatory cytokine in neurological disease or neurotoxicity. For example, we were surprised to find that mice lacking both TNF-α receptors, while protected against the dopaminergic neurotoxicity of MPTP, were rendered vulnerable to hippocampal damage by MPTP (Sriram et al. 2006a). Thus, very unexpectedly we found that TNF-α appears to be neurodegenerative in the striatum but neuroprotective in the hippocampus (data not shown), findings suggestive of starkly different region-dependent roles for this and perhaps other cytokines in the CNS. These observations also should give pause to the use of therapies to reduce or neutralize TNF-α in the brain, as treatments that might be beneficial to one region may be detrimental to another region.
Together, the data I have presented give you an idea of the need for more research to fill a number of data gaps. For example, we need to have a clearer understanding of the neuroinflammatory responses at the cellular and molecular levels. This reinforces the notion that new ligands for imaging neuroinflammation are needed; Dr. Avi Nath already raised this possibility in his presentation. With that prior molecular and cellular knowledge in hand, we can, of course, begin to delineate targets for neuroprotection, mindful of region-specific roles for a given neural cytokine/chemokine. Finally, we need to more clearly define risk factors/modifiers that contribute to or ameliorate the adverse effects of neuroinflammation.
D. Miller
I come at this from a different perspective. I am not a researcher in the area of NeuroAIDS, but I do have the perspective of someone who has been interested for a long period of time in how chemicals damage the brain and the type of factors, or cofactors that can have an impact on that neurotoxicity or damage. One of the susceptibility factors that I am quite interested in is chronic stress and how it affects neurotoxicity.
In reviewing the literature, it is quite evident that stress is often discussed in conjunction with AIDS (e.g., Capitanio et al. 1998; Kopnisky et al. 2004; Kumar et al. 2003). For example, social stress impacts the progression of AIDS; it impacts the morbidity and mortality associated with the disease. Now, stress is one of those terms everybody uses and each of us has their own definition of stress, but in general we can define stress as any event or happening that disturbs the homeostasis of the body. Of course, the body must deal with this disturbance.
Two main systems are brought into play in returning the body to homeostasis: the hypothalamic–pituitary–adrenal axis (HPA axis) and the sympathetic nervous system (SNS); and their secretory products, the glucocorticoids and epinephrine/norepinephrine, respectively (Miller and O’Callaghan 2002). The HPA axis and the release of glucocorticoids play a very crucial role in our day-to-day maintenance of homeostasis. When the HPA axis is not operating correctly, as in Cushings and Addison diseases, there are devastating consequences for health. Also, the HPA axis in concert with the immune system plays a role in combating bacterial and viral infections (Webster and Sternberg 2004). Interestingly, the adrenal gland appears to be a target organ in a subset of AIDS patients; some appear to have adrenal damage and endocrine alterations with possible adrenal insufficiency although the latter diagnosis is controversial (Eledrisi and Verghese 2001). Many studies report elevated cortisol levels in AIDS patients and antiretroviral therapy may exacerbate this (Freda and Bilezikian 1999). Interestingly, the HIV-1 genome contains a glucocorticoid response element that provides a possible means for cortisol to impact disease progression (Kumar et al. 2003). It is also well known that glucocorticoids and norepinephrine/epinephrine affect cognition as well (Miller and O’Callaghan 2005). Furthermore, some types of stress can result in a release of dopamine, another brain chemical that is known to play a role in the progression of AIDS dementia (Koutsilieri et al. 2002). Excessive activation of the SNS can enhance viral replication (Sloan et al. 2006). All of the above suggest stress and its associated chemicals could play a role in the development or progression of NeuroAIDS, as it does in AIDS itself.
As I mentioned, my laboratory has been investigating how stress and stress-associated chemicals such as the glucocorticoids impact the damage or neural injury caused by neurotoxicants such as kainic acid (e.g., Benkovic et al. 2004). When mice are treated with kainic acid in vivo, there is extensive damage to hippocampus as shown by the cupric silver degeneration stain and an activation of glia. We use astrocyte activation as a marker of damage, and an up-regulation of the major filamentous protein of astrocytes, GFAP as measured either by ELISA or immunohistochemically, is indicative of injury. Kainic acid induces a quite profound increase in GFAP in the hippocampus compared to saline (Fig. 7A and C). We also find apparent microglial activation after kainic acid as indicated by lectin staining (Fig. 7E and G). More important for our discussion, when animals are implanted with corticosterone pellets for a significant period of time, an experimental method designed to mimic extreme chronic stress, and then give kainic acid, their basal levels of GFAP are quite down-regulated, and we also lose the activation of astrocytes instigated by kainic acid (Fig. 7D). The microglial response is also inhibited (Fig. 7H). Other direct measures of damage, including Fluro Jade and cupric silver degeneration stains, suggest that corticosterone greatly reduces kainic acid-induced damage and subsequent neuroinflammation. However, these findings also suggest that stress chemicals influence the cells involved in the neuroinflammatory process; furthermore, there may be differences in how glucocorticoids impact the neuroinflammatory process initiated by means other than a chemical neurotoxicant. It is not known if the HPA axis changes that can occur in some AIDS patients contribute to the development of NeuroAIDS.
In terms of research needs, as it appears AIDS can alter the function of the HPA axis and we know that glucocorticoids are endogenous regulators of inflammation, including neuroinflammation, it would be useful to know if disruptions of the HPA axis play a role in either the initiation or progression of NeuroAIDS and the impact antiretroviral therapy may have on these. There appears to be little examination of the role of or the status of the HPA axis in NeuroAIDS. It would also be useful to examine these issues by utilizing animal models. It would be important to know the impact glucocorticoids as well as other stress chemicals such as adrenaline/noradrenaline and dopamine have on the disease process as well as their interaction with antiretroviral therapeutics. Studies should examine the effect of stress and stress chemicals on the ability of the virus to invade the CNS and determine if they impact the degree of viral load in brain and the ability of the virus to maintain a presence in brain. In addition, studies should determine how stress affects the neurological and neuropathological sequelae associated with the presence of the virus in brain.
B. Cox
I will address the role of smaller peptides in neurotoxicity in general, and I will begin by pointing out, as many others have, that in several situations with neuronal damage the neurotoxic agents are often small peptides. One example is β-amyloid(1–42), which is clearly toxic as a monomeric peptide; it is possible that the aggregation of β-amyloid(1–42) is one way in which the toxic peptide is segregated from neural contact and thus inactivated.
While some neuropeptides are potentially neurotoxic, in other cases there is evidence that they are protective. For example, the activation of the delta opioid receptor (the DOP receptor) by drugs or enkephalins has been shown to mimic the protective effects of cardiac preconditioning in models of myocardial infarction and may also have neuroprotective effects in stroke. Neural peptides, being critical regulators of neural function, have the potential to be either neuroprotective or neurotoxic.
I would like to draw attention particularly to one of the opioid peptides, a novel peptide nociceptin/orphaninFQ (abbreviated to N/OFQ for convenience; it has two names because the two groups who discovered it couldn’t agree on the terminology). This peptide has an amino acid sequence that shows similarities to that of dynorphin A. The critical difference is that the first amino acid in N/OFQ is phenylalanine, whereas in dynorphin A the first amino acid is tyrosine. This difference confers selectivity for the NOP-type of opioid receptor (also known as the ORL-1 receptor), which has significant sequence homology with the kappa type of opioid receptor (KOP-r).
Our interest in N/OFQ, which is quite widely distributed in brain, followed from our observations a few years ago that neurons and/or glial cells will synthesize N/OFQ in response to a number of stimuli, including neuronal depolarization, exposure to ROS or inflammatory mediators—each of which have been shown to be associated with various forms of neural injury. About the same time, another group showed that neonatal white matter lesions similar to those seen in periventricular leukomalacia (PVL) induced in neonatal mouse brains by ibotinate were exacerbated by coadministration of N/OFQ into the cerebral ventricles, and reduced by N/OFQ antagonists (Laudenbach et al. 2001).
The expression of N/OFQ is increased around the site of a simple mechanical injury to the brain (Witta et al. 2003). Following the insertion of a sterile needle 2 mm into the cerebral cortex of anesthetized rats or mice (under anesthesia, of course), there is a reactive response surrounding that injury. In contrast, the level of N/OFQ is much lower in the noninjured cortex. When you look at higher power with autoradiography in situ hybridization, you can see clusters of silver grains indicating N/OFQ gene expression overlaying markers of neurons, indicating that N/OFQ expression is specifically increased in neurons in this model.
The evidence that N/OFQ expression might be associated with neural injury lead us to evaluate possible roles of the peptide in Parkinsonian-like syndromes. There are several useful animal models of Parkinson’s disease (PD), as Dr. Hong has pointed out, of neuronal injury in general, and it may be particularly relevant to this meeting since HIV infection of the nervous system is one of many toxic insults that may produce Parkinsonian symptoms.
MPTP is a particularly potent dopamine neurotoxicant. We evaluated the role of N/OFQ in MPTP injury by measuring the effects of MPTP in mice with a genetic deletion of the N/OFQ gene, in comparison with the same treatments in wild-type littermates expressing N/OFQ normally. MPTP reduces the number of TH-positive dopamine neurons in both types of mouse, but in the N/OFQ knockout mice significantly more of the TH-positive neurons survive. In repeated experiments, we get about a 50% protection of the dopamine neurons against MPTP toxicity in N/OFQ knockout mice relative to wild-type mice (Marti et al. 2005; Brown et al. 2006). If you look at DA markers such as TH or the vesicular monoamine transporter (VMAT-2), in the striatum, the region in which axons from the substantia nigra neurons terminate, a similar picture emerges. Again, deletion of N/OFQ expression reduces the loss of DA markers induced by MPTP treatment (Marti et al. 2005).
Further evidence for the role of N/OFQ in modulating the behavior of midbrain DA neurons comes from our colleagues in Italy, Michele Morari and Giro Caló at the University of Ferrara. They have shown very convincingly that N/OFQ antagonists reverse motor impairment in several models of Parkinson’s disease (Marti et al. 2005). Thus pharmacologic blockage of NOP receptors reverses symptoms; genetic deletion of the peptide provides neuroprotection.
The mechanism of N/OFQ action remains unclear. It is possible that sensitivity to N/OFQ facilitation of toxicity is related to expression of the NOP receptor, since 50% of the substantia nigra dopamine neurons express the NOP receptor, but we have not yet fully explored that possibility. Morari and his colleagues have shown that injection of N/OFQ causes a significant increase in glutamate concentrations in the substantia nigra reticulata (SNr), and also observed elevated glutamate levels in SNr after haloperidol treatment, which also produces symptoms of PD (Marti et al. 2002). Expression of N/OFQ in SNr is also increased after treatment with 6-hydroxydopamine or with MPTP, suggesting that N/OFQ expression in this region is increased after damage to DA neurons and raising the possibility that the elevated levels of N/OFQ in this region are responsible for the elevated levels of extracellular glutamate. The location of the elevated levels of N/OFQ appears to be neurons in the SNr since the N/OFQ is colocalized with a neuron-specific marker, but we cannot rule out the possibility that there is also an increased expression of N/OFQ in local astrocytes and/or microglia because these cells can likewise synthesize N/OFQ.
Collectively, these results suggest that under conditions of neuronal insult with a toxin that causes injury by multiple mechanisms, including induction of ROS, excitotoxicity, activation of microglia, and liberation of a complex mixture of cytokines and neurotrophic factors, it is possible for the endogenous neuropeptide, N/OFQ, to play a deleterious role, thereby facilitating permanent loss of the damaged DA neurons.
I do not wish to suggest that N/OFQ is unique in this type of action. N/OFQ is an interesting peptide, but I am sure that there are other endogenous peptides acting through different, but related, receptors that will either be neuroprotective or neurotoxic, and their roles as protectants or toxicants may vary in different parts of the brain in response to different stimuli. What is needed now is a peptidomics approach to the analysis of toxin-induced changes in the expression of many neuropeptides in discrete brain regions. The toxic insult may be in mechanical, chemical, such as MPTP, or viral such as HIV. I suspect we will see a lot of commonalities in comparisons of different insults, but we should also expect regional differences across the brain because the local mix of released neurotransmitters and modulators will differ from region to region. We also need to determine the source of the released peptides. Is it neurons, astrocytes, or microglia? Potentially, each of these cell types might be a source of agents mediating either neuroprotection or neurotoxicity under different circumstances.
Neurotoxic peptides probably do not act directly to kill neurons, but by facilitating excitotoxins such as glutamate, or by liberating complex mixtures of cytokines and other cellular regulators. Additionally, there are potential interactions through iNOS, through COX, and/or TNF-α, which are clearly regulated by stimuli causing neural injury. The receptor target of the peptides mediating these effects and the signal transduction systems also need to analyzed. The receptors are interesting because many of these are G protein-coupled receptors (GPCRs), which brings me to my second to last point. The modulation of the receptor function can be agonism, antagonism, or inverse agonism, and we need to look at all of those features in order to evaluate the relative roles of the endogenous agents that control these receptors. Finally, the kinetics of these phenomena need to be studied. What is the window for exposure to modulators of neuropeptide function in order to reduce the impact of neurotoxic stimuli? The opportunity for prevention of neural injury is likely to be limited to time points relatively early in the chain of events from insult to neural cell death and will vary significantly among different types of injury.
G. Hanson
Although I don’t work in the AIDS field, my research area is methamphetamine, especially as it relates to neurotoxicity. Of course, there is a significant concern relative to the interplay between the use of this drug and the potential damage that HIV or AIDS can cause. This work has been supported by NIDA for a number of years and I will hit some highlights that might be relevant to an interaction between METH toxicity and HIV infection. Also, I would like to acknowledge that most of this work has been done in collaboration with Dr. Annette Fleckenstein.
The mechanism of methamphetamine, its pharmacology, and what ultimately expresses as neurotoxicity involves the monoamines, particularly dopamine, although there may be a role for serotonin and norepinephrine. However, I will focus principally on dopamine as the prototypic monoamine system. The amphetamines have some unique properties in the way they work initially to affect the vesicles associated with the vesicular monoamine transporter (Fleckenstein and Hanson 2003; Riddle et al. 2002, 2005, 2006). They cause reversal of this transporter, so if there is dopamine that is accumulated in the vesicles, a person takes high doses of methamphetamine and that results in the spilling of the dopamine into the cytosol (Volz et al. 2006). This, in turn, reverses the plasmalemmal membrane transporter, thereby causing an enormous release of dopamine into the synaptic cleft and subsequently activating its receptor target.
There is abnormal accumulation of dopamine both in the cytosol within the terminal and in the extracellular compartment, and neuronal function may be compromised, threatening the neuron’s ability to survive (Hanson et al. 2004a, b). We have examined the transporters because we think they are really the secret or the target of what methamphetamine does not only pharmacologically but also toxicologically (Hanson et al. 2004a; Rau et al. 2006).
One of the targets we examined was the vesicular monoamine transporter, VMAT-2. This Western blot (Fig. 8) examined the total amount of transporter protein 1 h after a neurotoxic regimen of methamphetamine. We looked at synaptosomes, and then we broke the synaptosomes down into their two components: the heavy membranes and these would include the plasma membranes, which would include the attached VMAT-2; and the vesicular fraction, which is a cytosolic fraction and also would be rich with vesicles and associated VMAT-2 proteins. After methamphetamine, and the methamphetamine itself was washed out, there was a 50% drop in the VMAT-2 protein levels. In the purified vesicular fraction, there was this dramatic reduction in VMAT-2 and presumably in the associated vesicle. Methamphetamine causes these vesicles in the cytosol to break free and migrate out of the terminal. We are not sure where they go, but the result is an accumulation of cytosolic dopamine, which is normally packaged and sequestered within the vesicles. Dopamine is a very reactive molecule so it oxidizes and creates all kinds of free radicals inside the cytosol, which could be problematic for the neuron (Riddle et al. 2006).
Western blots of vesicular monoamine transporter-2 (VMAT-2) protein in whole synaptosomal fraction and after separation into its purified vesicle-enriched and synaptosomal membrane fractions 1 h after four administrations (2-h intervals) of 10 mg/kg/injection of methamphetamine (METH). *P < 0.05 vs. corresponding saline. Treatment with METH dramatically reduced VMAT-2 protein in whole synaptosomal, and especially in vesicle-enriched, fractions.
If you look outside the neuron with microdialysis and measure the formation 2,3-dihydroxybenzoic acid (DHBA) from salicylate, you see that methamphetamine is causing oxidation and likely extracellular free radicals. So this suggests that the methamphetamine causes some oxidative mischief both inside as well as outside of the neuron (Hanson et al. 2004a).
To determine the impact of that oxidative mischief, we examined the dopamine transporter, that is, the plasmalemmal transporter. Again, 1 h after a methamphetamine toxicity regimen and after washing the methamphetamine away so there is no residual methamphetamine, we see this dramatic reduction in the activity of the dopamine transporters. This effect suggests that something happened to this transporter that perhaps is linked to methamphetamine and its toxic impact.
We have discovered that methamphetamine causes these transporters to internalize and traffic inside the cell. Once they are inside, we believe that they are exposed to the oxidizing dopamine that I previously mentioned because of METH’s effects on VMAT and the associated vesicles. This oxidation then affects the dopamine transporter, a protein that is very sensitive to oxidation. It causes complexes and DAT dimers to form. This is likely an oxidative consequence (Baucum et al. 2004). As an indication of what this means, we did some Western blotting and looked at dopamine transporters. Here is the monomer Westerns; this is saline, and these are animals exposed to methamphetamine (24 h after drug treatment). DAT molecules have gone from monomers to dimers and perhaps also tetramers as well as forming a smear of complexes in between. This likely occurs because the dopamine transporters are oxidized. This is demonstrated because when we expose these tissues to a reducing agent, the oligomers and smears are reversed, suggesting that inside the cell after a METH treatment there is considerable oxidation affecting all kinds of protein, and complex formations that have functional consequences (Baucum et al. 2004). So what does this all mean? To show you a slide from Dr. Volkow’s work looking in humans, this is the dopamine transporter (using a PET scan analysis) in methamphetamine users and this is 1–2 years after methamphetamine, so they haven’t used methamphetamine for a while. There is a decrease in the dopamine transporter, suggesting that the nigrostriatal dopamine system has been damaged. When we compare the rats and humans, it appears that our preclinical models are somewhat predictive of what is going on clinically and the mechanisms that we are elucidating in rats also probably have relevance to METH addicts.
As a wrap-up, where do I think this should be taking us, what sorts of suggestions would I have? What I have presented today are immediate events, these are things that are happening within a matter of 1, 3, or 4 h after high-dose METH use. Furthermore, we can intervene in the eventual toxicity even later than that. If we give a dopamine uptake blocker as late as 8 h after methamphetamine, we can still block the sequence of damage that shows up after days or weeks. While what I have shown you is probably the catalyst, or the initiating events, there are other things that are going on. I actually thought Jim O’Callaghan and Diane Miller might talk about this, but there is a microglial response that seems to be important and expresses as late as 24–72 h after METH exposure that may be the final stage, the final death throes or damaging consequences to these neurons.
What is important relative to HIV? One of the important things has to do with transporters. What does HIV in AIDS do to transporter mechanisms since they are so critical in the methamphetamine toxicity when you put the two together, is there going to be some synergism that occurs? If the HIV itself causes problems with transporter function then this could make METH-induced toxicity even worse.
Another thing that I think is important is the time sequence. Knowing the time sequence is essential if there are events that occur rapidly after they take methamphetamine or after they get infected with HIV. Once these pathological events are initiated, then perhaps intervention is very difficult. But if there is an issue of hours or even days where you still might be able to intervene, sort of like with stroke where there is a hypoxic event, if you can treat soon enough, then you may be able to alter the toxic expression. I think those are things we need to know so we need to follow the temporal sequence out. Find out when things are irreversible, find out when you have to intervene, and the mechanism of intervention is probably going to be different. It certainly is going to be different with methamphetamine abuse; we know if we give a D1 or D2 receptor antagonist we can block toxicity at the time of methamphetamine exposure. If we give a D1 or a D2 antagonist 2 or 3 h later, it doesn’t block toxicity. If we give a dopamine uptake blocker, as I mentioned to you, we can treat as late as 8 h and we can still block toxicity expression. So what is going on here? We need to determine how to intervene effectively.
A. Nath
Will Maragos could not attend this meeting but was kind enough to send his slides. I am familiar with his work on Tat, hence I will walk you through these slides. Tat is an HIV protein that is actively released from HIV-infected cells and has been shown to be neurotoxic. The experimental paradigm used by Dr. Maragos includes the injection of Tat protein directly into the rodent striatum followed by methamphetamine 24 h later (Maragos et al. 2002). Methamphetamine is administered i.p. at a dose of 5 mg/kg and given every 2 h for a total of four injections and then at variable periods of time he processes the brain samples. If threshold levels of Tat and methamphetamine (which by themselves do not cause a decrease in dopamine) were combined, they produce a synergistic toxic response. If you look at the dopamine metabolites, you see the same type of pattern here with HVA being affected the most followed by DOPAC. Autoradiographic studies using a ligand for dopamine transporters in brain sections from animals treated with Tat and methamphetamine show that there is decreased binding in the basal ganglia. There is great binding for the dopamine transporters in the striatum of these untreated animals and those treated with methamphetamine and Tat alone. He also showed that the tyrosine hydroxylase enzyme that is important for dopamine production is also impaired with combined treatment with Tat and methamphetamine (Theodore et al. 2006b). Thus Tat and methamphetamine affect the dopaminergic pathway at several different levels. He further looked at GFAP immunostaining and showed that there is massive amount of gliosis that occurs both in terms of microglial cell activation as well as astrocyte activation upon combined treatment with Tat and methamphetamine (Theodore et al. 2006d).
The interesting thing in keeping with the theme of this meeting is that he looked at these inflammatory mediators and particularly TNF, and he is trying to build a story here that TNF itself may be an initiating factor in causing dysfunction of the dopaminergic system (Theodore et al. 2006a). The way he did this was to first show that Tat can, in a time-responsive manner, induce TNF which confirms what has been published in the literature by several groups already. But then, he takes TNF receptor knockout animals and administers Tat and methamphetamine together. He found that in the wild-type animals there was the expected decrease in dopamine. However, knocking out the TNF receptor prevented the damaging effects of Tat and methamphetamine on dopamine, implicating that TNF at least in part is contributing to this loss of dopaminergic system. Here, using in vitro experiments where neuronal cell death is the endpoint, he showed the same phenomenon, that pharmacological blocking of the TNF receptor blocked the combined effects of methamphetamine and Tat.
When he looked at cytokine analysis in these animals, he found that both MCP-1 and TIMP, which is a metalloproteinase inhibitor, were both elevated in the Tat + methamphetamine-treated animals (Theodore et al. 2006c). Minor elevations in ciliary neurotrophic factor, cytokine-induced neutrophils chemoattractant-3 and macrophage inflammatory protein-3α were also seen. An important role for MCP-1 was demonstrated when MCP-1 knockout mice failed to show a synergistic response to methamphetamine and Tat administration.
In summary, Tat and methamphetamine synergize to cause striatal dopaminergic loss resulting from dopamine terminal degeneration, and the mechanism of damage involves an inflammatory response induced by Tat.
Future questions that we need to address are: What is the role of TNF-α in dopaminergic terminal loss? For instance, does TNF-α activate iNOS and ROS formation, and other innate immune responses? Does TNF-α itself, through impairment of mitochondrial function, contribute to terminal damage? Dr. Maragos also wants to address the questions of how TIMP or MCP-1 can lead to dopaminergic terminal loss and whether MCP-1 increases infiltration of phagocytes. He also raises the possibility that macrophages may be a target of action of methamphetamine.
Discussion following session 2
J.S. Hong
Dr. Cox, I find your presentation very interesting. I wanted to add just a little bit of information, which we recently published, about a year ago, regarding the different kind of opiate peptides including all these peptides that you have mentioned on your slides. Interestingly, you list glycine–glycine–phenylalanine, which are three common amino acids in the N terminus of all the opiate peptides. We found that this tripeptide is a potent neuroprotector because of its anti-inflammatory effect. Pharmacophore analysis indicates that glycine–glycine–phenylalanine is almost identical to naloxone, which is also both anti-inflammatory and neuroprotective. However, both naloxone and the tripeptide GLY–GLY–PHE exert their neuroprotective effect independent from the conventional opiate receptors.
B. Cox
I don’t have any practical experience with these particular peptides so I can’t really comment.
G. Hanson
Both Dr. Hong and Dr. Cox talked about the opioid peptides, what about other peptides that are associated with some of these systems such as substance P, which is an inflammatory mediator, and neurotensin, which has been associated with inflammation as well? Is there any role for these other peptides?
J.S. Hong
We have studied other peptides, such as substance P. Substance P is one of the most important proinflammatory peptides. In subpicomolar concentrations, this peptide is neurotoxic to dopaminergic neurons in rat midbrain neuron/glia cultures (Block et al. 2006).
C. Power
There is some precedence for up-regulation of various proteases in the context of HIV infection in the brain notably MMPs and trypsin. Is anything known about what regulates the cleavage of the pro-opioid peptides or substance P, and is that regulated by inflammation?
B. Cox
Intraneuronal processing occurs in vesicles where there are enzymes such as proconvertase-1 (PC1) that cleave precursor peptides at intramolecular peptide bonds following pairs of basic amino acid residues (e.g., Lys–Arg or Lys–Lys), which appear to serve as a signal for intramolecular cleavage. This type of precursor processing to mature peptide occurs in neurons. What is not known is how the precursor peptide is processed in glial cells. It is not clear that the same vesicular machinery is present. Nor is it clear how peptides get out of the glial cells; whether it is via vesicular release or endocytosis, or whether there are other kinds of processes. We’ve looked a little bit at this in vitro in cultures of astroglial cells, where it appears to us that the peptides are released as the precursor peptide or as only partially cleaved fragments of the precursor. When you increase the synthesis of the precursor peptide mRNA leading to precursor peptide synthesis, you get an almost immediate release of the intact or partially processed precursor peptide to the extracellular medium. Whether the released larger peptides can then be further processed outside the neuron to yield “mature” neuropeptides is also another question worth looking at.
J. McArthur
This question is for anyone who has had experience with the in vitro models and most of the model systems that we have seen today have been acute or hyperacute models of synergism between tat or HIV proteins and drugs of abuse. Are there chronic models that we could use, or that have been used, that might mimic better the situation in humans?
G. Hanson
Those kinds of models require months, if not years of exposure. So if we wanted, let’s say methamphetamine or any of the drugs of abuse, if we wanted to mimic what is going on in humans, the exposure is going to be over a long period of time. The doses need to be escalated, usually the addicts are polydrug users so there should be multiple substances that are involved and then you introduce the HIV or related proteins later on to see how they interact with this system that has already been compromised or altered.
J. McArthur
It is important to consider the differences between the in vitro studies showing the synergistic effects of drugs of abuse and HIV proteins and the clinical cohorts that have really not appeared to show these differences.
W. Royal
It is probably worth mentioning that Dr. Robert Donahoe at the University of Utah has a model of chronic opioid exposure in macaques.
G. Hanson
One of the things with methamphetamine is this issue of tolerance. Tolerance definitely does occur in humans and it occurs in the animal models, and we typically don’t look at the tolerant animal in combination with HIV.
M. Carson
Carol Colton’s work has been very informative when we are looking at free radicals, nitric oxides; it is very different between rodents and the higher primates. I was curious as to what has been translated. It seems to be substantially different, due to substrate limitations, differences on how dopamine decarboxylase is regulated, some of these other things that feed back.
J. Berman
Well, the only useful model, so far, other than some animal models, are these two patient cohorts that we have that are so large. The patients have been infected for somewhere between 5 and 7 years, and are very well characterized in terms of their substance and alcohol abuse. We have begun to look at their PBMCs, and they revisit every 6 months so we can look for changes as compared to acute infection that we mimic in vitro because we can’t get the patients when they are acutely infected.
W. Royal
Is there a known clinical correlate for inverse agonism?
B. Cox
Inverse agonism is easiest to demonstrate in vitro with cultured cell preparations and by manipulating the levels of receptor expression, but there is certainly evidence in functional assay systems for some GPCRs that drugs that act as inverse agonists produce the opposite functional effects to those of the conventional agonists. This probably occurs because there is inherent basal activity in the receptor–G protein complex even in the absence of bound conventional agonist. This makes it possible for another receptor ligand to favor receptor conformations with less basal activity than those conformations that are present in the absence of ligand. Several clinically used drugs acting through GPCR appear to be inverse agonists; one example is cimetidine acting at histamine H2 receptors where it reduces histamine effects by inverse agonism.
Dr. Hanson also asked about other kinds of peptides, and whether there was something unique about opiates. We happen to call them opiates, but they are just one of a hundreds of neuropeptides. They are a class of peptide that acts through GPCRs and there are many other GPCRs that might also modulate neurotoxicity. One example is the adenosine A2a receptors, where caffeine is an antagonist. Caffeine is an effective neuroprotectant against loss of dopamine neurons in some experimental models of Parkinson’s disease, suggesting that endogenous adenosine might also play a role in disease progression. Clinically, there is indirect evidence that nicotine or tobacco smoking may reduce the incidence of Parkinson’s disease, raising the possibility that nicotinic receptor activation can provide some relief. These observations suggest that it is just a matter of manipulating the right neurotransmitter or neuromodulator receptors to provide some degree of neuroprotection. It matters what is expressed on what cell in the neural networks regulating dopamine neuron function. The critical question becomes—where are the receptors that offer protection located? We are going to have to map in detail the neural and/or glial locations of the critical receptors and of their endogenous ligands to determine the most effective way to provide protection against neurotoxic drugs or other insults. This is probably as important for those studying glia as for those working on neurons since, as others have pointed out, an astrocyte in the frontal cortex is not the same as an astrocyte in substantia nigra or striatum.
J. Berman
With regard to GPCRs, we showed that CCL2 is neuroprotective from apoptosis. In the theme of the talk we have heard about TNF and other factors, depending on when they are expressed, and where they are expressed they can promote inflammation or protection. CCL2 is another molecule that binds to GPCRs and has those effects.
H. Fox
There are data on SIV and HIV causing dopaminergic deficits. We have Joan Berman now showing us heightened dopamine in the periphery of drug abusers and HIV-infected individuals. There is recent data in, my favorite journal, the New York Times, about Parkinsonian patients on l-DOPA who develop compulsive or addictive behaviors, gambling, and probably other ones. Is there a similar action here as far as behavioral effects? So dopamine goes down in the brain because of HIV, let’s say, you take methamphetamine to raise your dopamine, which I believe leads to an endless cycle. Is there some self-treatment of patients, potentially interacting with the drug abusing or addictive behaviors? Does that make any sense?
If dopamine goes down in the brain because of HIV and maybe up in the periphery, but of course it doesn’t get through because of the blood–brain barrier, and patients develop Parkinsonian-like syndromes. If you take methamphetamine or cocaine, maybe it will improve at least for a while. Just as people with psychiatric disorders who self-medicate with drugs, does it impact on addictive behaviors, which are dopamine-driven?
L. Chang
I was involved in a conference in Thailand a few years ago because in Thailand, methamphetamine abuse was a very big problem, especially in the North where drug use is rampant. This was before the government introduced the program that put everybody on antiretroviral treatment. Locally, what the physicians reported was that the HIV patients, especially those with dementia, would take methamphetamine in order to “feel better.” They were self-medicating in a sense. Those patients in the local villages were not treated with HIV medications, so they quickly developed moderate to severe stages of dementia and they would then take methamphetamine for self-treatment. There was actually a pilot study that Charlie Hinkins did at UCLA, using methylphenidate to treat HIV patients, and they showed some improvement in cognitive performance, but we don’t know what happened chemically or biologically in the brain, but clinically they had some mild improvement.
A. Nath
What happens to the amygdala in HIV-infected individuals?
H. Fox
We haven’t looked, a major miss.
B. Cox
Just to come back to the question of self-medication, the usual explanation of why cocaine addicts binge on cocaine is that dopamine levels (in the nucleus accumbens and elsewhere) goes up acutely after each cocaine dose but then go down as the cocaine blood levels fall. The assumption is that the need to take additional doses of cocaine is driven by an attempt to keep the dopamine concentration in critical brain regions above the initial dopamine level, but this becomes progressively more difficult as the release of dopamine with each additional dose of cocaine is reduced and the psychoactive effect of the drug becomes progressively diminished. Eventually, other toxic actions of cocaine intervene, or exhaustion makes it impossible to continue to take more drug.
J. McArthur
Since we have so many people with expertise in microglia and we probably have introduced this concept of regional localization of HIV replication, is there something special about the microglia from simple things such as density, within the basal ganglia structures within the striatum?
M. Carson
There is a lot of literature out there that’s not been pieced together that you can really see acute differences in responses to cytokines and other things in these regions. What we can really see is that the microglia in the thalamus in general are very different than all the rest of the brain. Then what we see is that in some of those regions, there is a lot more microheterogeneity. That area seems to require more specific, specialized help is how I read it—they are instructing a lot more specialized behaviors. If you can enlighten us with what the means, that would be fabulous. We are trying to put this out there as a survey, because we can only study subsets.
J.S. Hong
Yes, I can add a comment about the heterogeneity. We reported a few years ago that in the substantia nigra, the density of microglia is about five times higher than the rest of the brain (Kim et al. 2000). This is probably the reason why a single systemic injection of LPS causes progressive loss of dopaminergic neurons in the substanita nigra.
G. Hanson
This partially answered my question, but the other part of that is what does the striatum look like, since the nigrostriatal dopamine system is so vulnerable to all kinds of toxicity.
J.S. Hong
I can probably partially answer your question. We injected LPS into two different regions. One is a nigral injection and the other one is an intrastriatal injection. The nigral injection is very effective in killing dopaminergic neurons. However, intrastriatal injection of LPS is also capable of damaging dopaminergic neurons, except it requires higher doses and takes a longer time to kill neurons.
E. Masliah
I think that you also have to consider, when you are talking about regional differences and actually microglial differences, trafficking of a monocyte/macrophages to these different regions and the role of the permeability of the blood–brain barrier in all these different regions. I know Monica Carson talked a little bit about differentiating macrophages and microglia and how valuable this is or not, but at least primarily from the point of view of HIV, I think that we really need to think not so much to the rest of microglia, but also the trafficking. I don’t know if you see more trafficking to the basal ganglia or the substantia nigra.
M. Carson
Those LPS studies are really very interesting with the injections. We are using about 50,000-fold lower doses of LPS than are usually used and we get a very inflammatory model, which just tells you something. But LPS is perhaps not the most representative pathogen going to the Toll-like receptor 4. What we find is that when we used some of the other ligands for some of the other pattern recognition receptors or other Toll-like receptors, we get different results. Different microglia are responsive to different areas. Specifically, I think this goes to the HPA axis issue. We’ve done things where we’ve caused an acute adjuvant or autoimmune response. It is very specifically in the key places of the hypothalamus, and then you watch, in the neurons first, then you see it coming up in the microglia there, then you see this wave propagate through specific regions. Again, neurons driving the glial response, appropriately or inappropriately, are a key thing to keep in mind. The other thing we see, depending on the amounts of macrophages that come in, is you get a very significant different outcome on neuronal survival. I think that really goes to trafficking and it can be caused by leakage of blood–brain barrier, by expression of chemokines in the presence of an intact blood–brain barrier, there are a lot of issues.
Session 3: NeuroAIDS and virology
J. McArthur
I am going to present a brief clinical review of some of the neurological complications. Obviously, this morning we’ve been mainly focusing within the CNS, and Chris Power and I are going to move down to the peripheral nervous system. I will hopefully highlight some of the opportunities for research in that part of the neuroaxis. I am going to review briefly some of the clinical features of sensory neuropathies. Peripheral neuropathies are a growing neurological problem both here in the United States and in resource-limited countries. I will briefly talk about pathology and pathophysiology, as it relates to the human situation.
First, this is a quote from a patient of ours who had a painful peripheral neuropathy linked to HIV infection: “springtime in nerveland.” I think it is a very good description that summarizes the phenotype of this neuropathy, with burning pain in the feet. This sums up exactly what patients experience. This can be a very disabling problem; although it does not affect survival, it can certainly affect the quality of life. More importantly, the development of neuropathy may affect compliance with antiretroviral medications. The symptoms of painful neuropathy in the context of AIDS include both spontaneous neuropathic pain, where pins and needles sensations, tingling, stabbing pains occur spontaneously, and also evoked pain that is stimulated by touching, rubbing, standing, or exercise. The examination features suggest that this is an unusual neuropathy because it is primarily a sensory neuropathy. So, unlike some other types of neuropathy—for example, diabetes or the inflammatory neuropathies such as Guillain–Barre—this is almost exclusively sensory. This suggests that there may be a selective vulnerability of sensory nerves and the dorsal ganglia to the effects of HIV.
Importantly, from a clinical perspective, as we evaluate patients who may have sensory neuropathy associated with HIV, there are a number of confounding illnesses or conditions which complicate assessment. The first is the metabolic derangements of HIV infection and antiretroviral use. In a survey in our own clinic, about 11% of HAART recipients develop frank diabetes mellitus and about 20% have impaired glucose tolerance. Thus it is very clear that patients with HIV may also be at risk for developing metabolic consequences that can contribute to peripheral neuropathies.
Alcohol abuse and hepatitis C both have been associated in a magnifying role with the development of peripheral neuropathies. Then there are a number of other conditions, for example, entrapment neuropathies, where nerves are bruised or entrapped as they cross bony prominences. Vitamin deficiencies or overuse of specific vitamins such as B6, and various musculoskeletal conditions can mimic some of the features of sensory neuropathy.
Now, from an epidemiological point of view, the prevalence of HIV sensory neuropathies is rising. If we think about 1996 where the prevalence was about 20% in our clinic at Hopkins, we have actually seen a doubling in the prevalence of HIV-associated sensory neuropathies despite the widespread use of HAARTs. There are a number of identified risk factors for sensory neuropathies: older age is associated with a 2- to 3-fold increase risk of developing neuropathy, diabetes mellitus, and impaired glucose tolerance. In general, the lower the CD4 count and the higher the plasma HIV RNA levels in the early phases of HIV infection, the more likely an individual is to develop neuropathies. There is clearly a link between viral replication and immunological damage and the subsequent development of neuropathy. There have been a number of genetic risk factors for neuropathy and the ones that have been identified to date include APOE-4, which appears to be a potent genetic marker for development of neuropathy and certain mitochondrial DNA haplotypes.
What is less clear is the exact risk with which antiretroviral regiments produce toxicity within the peripheral nervous system. Our own studies in collaboration with a group in Melbourne suggest that use of nucleoside reverse transcriptase inhibitors ddI and d4T is associated with somewhere between and 3- to 8-fold increased risk of developing neuropathy. This appears to be a very specific neurotoxic effect of these antiretrovirals. The continued relevance of this field of NeuroAIDS is that around the world, these drugs are still very widely used. For example, d4T is used in about 60% of generic fixed-dose combinations worldwide. Resource-limited countries often manufacture their own generic antiretrovirals and these frequently contain d4T. Thus worldwide, d4T neurotoxicity continues to be a problem.
In terms of pathology, I have just summarized here some of the pathological features that we see and identify within the peripheral nervous system. I would emphasize that there is a lot of commonality between the peripheral nervous system pathology and the central nervous pathology. Within the peripheral nervous system, HIV infection is confined to perivascular macrophages, primarily within the dorsal ganglia and the proximal nerve trunks. There is prominent macrophage activation and a lot of the talk this morning about CNS, cytokine and chemokine activation and overexpression certainly pertains to the peripheral nervous system. From an ultrastructural point of view, the Remak bundles, which are the unmyelinated C fibers, the fibers that subserve pain, are damaged and the density of these fibers is decreased. Structurally abnormal mitochondria can be identified in patients who have received ddI and d4T, and who have developed neurotoxicity from these antiretrovirals. The mechanism of damage to the peripheral nerves is still being worked out, but the end result is that there is a length-dependent loss of both cutaneous and centrally directed nerve fibers. Although this is primarily a painful neuropathy, ultimately both small- and large-caliber nerve fibers are affected.
The skin biopsy of a patient with HIV sensory neuropathy has a normal-looking density within the epidermis to the top of the panel, the dermis at the bottom. This particular stain is with a panaxonal marker PGP9.5. Our group and others have used skin biopsies as a very useful marker of the degree of damage of epidermal nerves.
To summarize some of the things we know about the pathogenesis of HIV neuropathies, it is associated with advanced HIV disease. The initial CD4 count and baseline plasma HIV RNA predict which individual is at higher risk to develop neuropathy. The beneficial therapeutic effect of antiretrovirals on neuropathies is poorly documented. Avi commented on the rather dismal state of our knowledge base for dementia trials. We also know relatively little about the affects of antiretrovirals in the peripheral nervous system. One study from 2000 looked at antiretroviral recipients and individuals who showed a virological response in terms of plasma HIV RNA also had some responses in their thermal thresholds. Apart from these rather meager data, we know surprisingly little about antiretroviral effects within the PNS.
As Avi Nath mentioned this morning, the toxic effects of HIV proteins, tat, and gp120 have been studied not only in cerebral context but also in dorsal root ganglia (DRG) cultures, and it is known from work by Richard Miller at Northwestern University that some of the HIV proteins (gp120, for example) can bind to chemokine receptors within the DRG and induce calcium fluxes and neuronal injury.
Finally, our own group led by Carlos Pardo has quantified the degree of macrophage activation within DRG and showed a clear association with symptomatic sensory neuropathies. Again, macrophage activation plays a clear major role here, as with the CNS.
I am going to conclude there. I think the three areas for research opportunity that I see ahead of us are as follows: (1) epidemiology—we need to define not only the genetic but probably the metabolic risk factors for neuropathy; (2) mechanistic—what is actually going on to cause this length-dependent denervation process within the peripheral nervous system; (3) the treatment aspects which up until now have focused primarily on symptomatic treatment. We clearly need more emphasis on regenerative strategies that could allow for damaged nerve fibers to regrow.
C. Power
It has been an exciting meeting and it is interesting for me to meet with people from different disciplines. I am going to talk about polyneuropathy and HIV infection, and I think it is very relevant to this group for a couple of reasons: (1) It is common among patients with HIV infection, and (2) the treatment options are limited, and there is a great potential for drug abuse in that one of the standard treatments for neuropathic pain includes opiates as well as other therapies such as the anticonvulsants. So, there is clearly an interface between polyneuropathy and drug abuse. It is clear that polyneuropathy as well CNS disease has a substantial impact on the quality of life and cost of care among patients with HIV-related neurological disorders as illustrated with polyneuropathy as well as cognitive impairment, as recently shown by our group (Pandya et al. 2005). Interestingly, the cost of neurologic disease appears to anticipate the actual diagnosis, in that the costs rise before the diagnosis is established.
In terms of the underlying mechanisms of HIV-related sensory neuropathies, there are really just two major groups. There is the distal sensory polyneuropathy (DSP), which is presumed to be caused by the virus itself, as well as the antiretroviral therapy toxic neuropathy (ATN). We were initially interested in what kind of virus one could find in the nerves of patients with and without neuropathy, and in this collaborative work we did with the Hopkins group—to make a long story short—we found that we could certainly find virus in the nerves of those patients with HIV-related neuropathy as well as those without neuropathy, but the viruses were quite distinctive from brain-derived viruses in the sense that they were closely related to those viruses found in the blood of the same patients, and the viruses used both CXCR4 as well as CCR5. In fact, we found some dual tropic viruses as well. This is different from what is found in the brain, as most viruses identified in the brain are CCR5-dependent.
We have developed a model in the laboratory using a hCD4/CCR5 transgenic rat. It is a model that expresses human CD4 and CCR5 receptors for HIV on monocyte cells as well as lymphocytes, and in fact these are illustrations that one can find HIV infection in cultured DRG from these animals that have been infected with HIV. It is clear that the virus does infect cells of monocyte lineage, notably those ED-1-positive macrophages as shown here. These cultures are composed of neurons, macrophages, and Schwann cells, which are GFAP-positive. Using this culture system, we then asked the question: how do those viruses that were isolated from nerve behave in vitro? We constructed recombinant viruses containing the HIV envelope derived from nerves of patients with neuropathy. We were not able to clone the whole virus, but cloned out the envelope sequence, or part of it, and then made a recombinant virus, which was infectious. Most of these viruses were CCR5-dependent, but when we infected the cultures and then examined neuronal injury as measured by neurite length, we found that there was evidence of neuronal injury and indeed also neuronal death, as illustrated here. The viruses exerted some type of neuropathogenic effects.
We have also used this model to look at another question. For some time I was suspicious that in fact it was not just the conventional “D” drugs that caused peripheral neuropathy, ddC, ddI, or D4T, but actually some of the other antiretroviral drugs also exerted neurotoxic effects on the peripheral nervous system. We found this to be the case as part of a larger epidemiological study; in effect, we found that indinavir was neurotoxic in vitro in conjunction with HIV infection. Again, these experiments were performed in the rat DRG cultures that I mentioned to you a moment ago. These are healthy cultures with long neuronal processes or neuritis, but after one infects with HIV, the cultures show HIV p24 immunoreactivity. Moreover, there are fewer neurons, and their processes are much shorter. We compared that here showing that HIV reduces the neurite length as does indinavir alone, but when you put the two of them together, you actually get an additive neurotoxic effect as illustrated here. Not surprisingly, ddI was also neurotoxic in this system. So this is an in vitro system by which you can look at mechanisms underlying the development of polyneuropathy.
We also wanted to ask the question in vivo, and to do this we used a model of feline immune deficiency virus (FIV) infection. It is a lentivirus like HIV that causes immunosuppression and neurologic disease in cats. It uses CXCR4 and CCR5, and now there is a primary receptor, CD134, that appears to be important also. These cats develop encephalopathy, and they also manifest electrophysiological changes compatible with neuropathy. We wanted to determine if they developed peripheral neuropathy, and in fact they do. The animals exhibited delayed responses to a noxious stimulus, notably a thermal stimulus during the course of infection, relative to the control animals. Using the skin biopsy assay that Justin McArthur mentioned that was developed at Hopkins, we also looked at the pads of those animals with FIV versus the control uninfected animals, and in fact there were far fewer processes or nerves in the epidermis compared to the controls. In fact, we looked at this over time, and at 8 weeks the FIV+ and FIV− animals were about the same, but by 12 weeks postinfection, it was clear that there were far fewer nerve processes in the FIV infected animals’ footpads compared to the FIV− controls.
So, with that thought in mind, we again wanted to develop an in vitro system as well. Essentially, we did the same thing that we did with the rats. We took DRG from healthy cats, cultured them, and then infected them with different strains of FIV, and we actually did this with different strains of FIV. Essentially, what we got is just as we saw in vitro with the rat cultures, reduced neurite length in the FIV-infected cultures. The neuronal size was reduced, and neuronal survival was likewise reduced in the FIV-infected cultures. This effect is dependent on the virus strain, and we are fortunate that we have a neurovirulent molecular FIV clone that we made in the laboratory because there are strains that do not appear to cause this. We pursued the disease mechanism further and it was evident that FIV infection of these cultures induced iNOS as well as STAT-1, as illustrated by Western blotting and PCR in the cultures and also in vivo from animals, showing that STAT-1 and iNOS are induced in the FIV-infected animals compared to the uninfected animals.
A topic that has received some attention in the literature is the role of T cells. If you look through the literature, it turns out that T cells are also invading the DRG and the nerves, and in fact, this also applies to the CNS. So we looked to see whether or not we could find CD3-immunopositive lymphocytes within the DRGs of FIV-infected animals, and in fact, they were very easy to detect. This is actually double labeling with a neuronal marker in the DRG, and you can see that there are lots of T cells. We counted them here and clearly show that there are more T cells in the DRGs from the infected animals. We then adapted our in vitro DRG system, and actually mixed syngeneically matched lymphocytes with DRG cultures; here is a T cell adhering to a neuron, and these are neurons with CD3-positive adherent lymphocytes, with a reduction in neurite length again as we had seen just with FIV infection of the DRG. When we used FIV-infected lymphocytes, this further accentuated the neurite damage and soma atrophy. Likewise for neuronal survival, the FIV infection of T cells appears to amplify neuronal death as shown here. I should say that we had to stimulate the lymphocytes in advance with phorbol myristate acetate or PMA. The PMA did not appear to contribute to neuronal death.
We wanted to pursue this a little further in terms of mechanism, and to make a long story short, it appears that when CD154 (or CD40 ligand) was suppressed on lymphocytes, there was improved neuronal survival. That is actually illustrated here and was chiefly due to CD8 cell-mediated injury. It was clear that the CD8 cells were mediating the neuronal damage and it seemed like it might be due to activation and expression of CD154. So what we think is happening in this system is that HIV or FIV infects macrophages, resulting in the activation of macrophages in the DRG. This likely results in the release of free radicals, and we have heard a lot about that already today; but in addition, this other finding may reflect a complementary or parallel system in which activated T cells enter the DRG where they contribute to neuronal death, possibly through a CD154/CD40 interaction.
E. Masliah
What I would like to do now is to bring the discussion back to the question of interactions between drugs of abuse and HIV. We have been primarily working on the interactions between methamphetamine and HIV in San Diego. As you all know, in California, methamphetamine abuse is a major problem, and a significant proportion of HIV patients are METH users. With respect to methamphetamine neurotoxicity, I think we focus too much on the degeneration of nigral neurons. It is important to say that there are other neural populations that are susceptible to the neurotoxic effects of HIV and METH. Specifically, we have found that calbindin-immunoreactive interneurons are highly sensitive to the neurotoxic effects of these two agents. Damage to calbindin neurons results in very significant memory deficits. The potential mechanisms through which HIV and METH damage calbindin interneurons is not clear, but recent evidence suggests that methamphetamine in combination with HIV has the capacity of inducing interferon-related pathways and specifically inducing interferon genes such as ISG15. We have studied the combined effects of HIV and METH in the brains of patients followed at the HIV Neurobehavioral Research Center by Dr. Grant and colleagues, as well as in transgenic mice challenged with METH and in primary neuronal cultures. We have observed that HIV patients with a history of METH use show more prominent astrogliosis and microgliosis in the white matter compared to controls. This is consistent with what Linda Chang described and with the work that Gil Gonzalez will present. However, the severity of the HIVE is lesser in METH users compared to non-METH HIV patients. In addition, and to make a long story short, the most significant damage was to the calbindin inhibitory interneurons in the frontal cortex, hippocampus, and basal ganglia. As you all know, the interneurons are producing gamma aminobutyric acid (GABA), and these are cells that regulate the firing of pyramidal cells. There is actually quite a bit of data showing that interneurons play a role in cognition, and that methamphetamine damages interneurons. We have also confirmed some of these observations in transgenic animal models. Actually, the combined effect of HIV proteins gp120 and methamphetamine results in an increased memory deficit in the transgenic mice. Let me just close by talking about the future or potential ideas as to what are the possible mechanisms. In primary neuronal cultures, HIV and METH damage mitochondrial function and increase calcium currents. Oxidative stress then plays an important role in toxicity. But in addition, we have recently found that induction of interferon-related genes might also be involved. We are currently investigating how HIV and METH trigger the production of interferons and how the interferon-related genes produced by astroglia might promote neuronal damage.
R. G. Gonzalez
I am going to discuss neuroimaging, and specifically a flavor of imaging known as magnetic resonance spectroscopy (MRS). We’ve been using it for several years to look at the effects of HIV on the brain in humans and in an animal model of NeuroAIDS. I’d like to first introduce MRS. Slide 2 has an MR spectrum that’s derived from the brain of the animal also shown on the slide. This MR spectrum from the macaque brain frontal lobe is very similar to a human brain MR spectrum. It appears relatively simple, but it is actually quite complex and informative. Where does this spectrum arise from? As shown on the slide, it was obtained from the delineated voxel, but that voxel is a complex mixture of neurons, oligodendrocytes, astrocytes, and other cell types. It turns out that if you separate all those brain cell types, and you perform MRS of each cell type, they would be different. Neurons have a spectrum distinct from astrocytes, and they are distinct from all other cell types. When an MR spectrum is acquired from a voxel shown on this slide, it represents a summation of individual spectra from each of the cells that are within the voxel.
This imaging tool is also a chemical analysis tool that provides neurochemical information noninvasively. One of the things that we’ve learned with MR spectroscopy in the last decade that we’ve been using it clinically, is that the response in the brain to a variety of insults is stereotypic in terms of the neurochemistry revealed by the MR spectrum. Diverse brain insults from trauma to Alzheimer’s disease to AIDS dementia result in a stereotypically abnormal brain spectrum. There is enlargement of the two peaks on the left side of the spectrum, which arise from several neurochemicals. Subsequently, in the course of the disease, a decline of the large peak on the right is observed. There are a couple of points I wish to emphasize about the MR spectrum. The peaks represent water-soluble, intracellular metabolites that are in the 1- to 10-mmol range in the brain. These neurochemicals are not metabolically static, and the spectrum depicts their steady-state levels. For example, NAA has a 100% turnover in 16 h, indicating a highly dynamic and biochemically very important process. I began investigating the macaque model because the biological basis of the human MR spectrum, despite detailed clinical information, is poorly understood. The primate model has a brain MR spectrum that is quite similar to the human spectrum, and has the advantage that the brain can be removed, and detailed chemical analysis can be performed.
The areas that are considered glial markers arise in the left-hand side of the spectrum and are commonly identified as the “choline” and “myo-inositol” peaks. The peaks appear deceptively simple, but they do not arise only from those chemicals. The resonances actually arise from several different biochemicals. These peaks are really quite complex and rather poorly understood, except that these changes occur in a variety of brain cell types in response to various insults.
An exception to the complexity of the MR spectrum is the large peak that arises on the right side of the spectrum and is designated in the slide as n-acetylaspartate (NAA). NAA is found almost exclusively (>95%) in neurons. It is thus a neuronal marker. Moreover, NAA decreases when you insult the neuron permanently or in a temporary fashion. In work we performed with Dr. Masliah few years ago using the SIV macaque model, we showed that the best neuropathological correlate of changes in NAA is changes in synaptophysin (Lentz et al. 2005). Basically, the level of NAA is a surrogate marker of the density of synapses. That is how we have been using and interpreting it.
Now I want to switch over to the animal model that we have been using for the last couple of years in collaboration with Ken Williams. It is a new and informative way to study SIV encephalitis in the macaque. The model involves the infection of macaques with SIV, and the depletion of their CD8+ T cells using an antibody. Remarkably, these animals develop profound encephalitis over a period of 8–12 weeks (Williams et al. 2005). The profound neuronal injury that we observe in this model is exemplified on slide 3. The two MR spectra on the left slide were obtained from an individual animal before and 8 weeks after SIV infection and CD8+ cell depletion. We can easily appreciate that there is a decrease in the height of the NAA peak. On the right-hand side of the slide, data are presented from four infected animals versus four controls. These data are from postmortem brain tissue, showing several neuronal markers. We found declines in synaptophysin, MAP2, and NAA/Cr.
This model rapidly produces profound neuronal dysfunction. Slide 4 depicts the results from an experiment in which we studied four animals that were SIV-infected and CD8+ cell depleted, and four similar animals that were treated with antiretrovirals. The black boxes on the graph represent the mean NAA levels of the four animals that were not treated and developed encephalitis. A significant, linear decrease in NAA was observed in the course of 8 weeks after infection. The next slide (slide 5, first column) demonstrates the typical histopathological changes found in the brain of these animals. We find hallmarks of SIV encephalitis, which is identical to HIVE, and includes large numbers of perivascular macrophages, multinucleated giant cells, and microglial activation. The slide also demonstrates abundant virus colocalized with the perivascular macrophages.
In a different cohort of four animals, combination retroviral therapy was begun 28 days after the start of infection. Surprisingly, we observed a rather dramatic reversal of the levels of this neuronal marker NAA as can be seen on slide 4. The time course of NAA in these SIV infected, CD8 cell depleted and antiretroviral treated animals are depicted by the open circles. Antiretroviral therapy was begun 4 weeks after infection. We found that 4 weeks after the start of antiretroviral therapy, NAA returned nearly to baseline levels. Histochemical analyses of the brains from these animals are shown in the second column of slide 5. The brain appeared nearly normal histologically with the exception of a few scattered perivascular macrophages. We were unable to identify the virus in the brains of these animals. The rapidity of the reversal was quite extraordinary. These observations within the brain were particularly interesting when considered in contrast to what was occurring outside the CNS.
We measured plasma viral loads in these animals, and there was only a minor decline in the viral loads in animals treated with antiretrovirals. However, we did observe that a specific subset of monocytes declined. In nontreated, SIV-infected, CD8+ cell depleted macaques, we observed an expansion of the specific subset of monocytes that are CD14+ and CD16+. In antiretroviral-treated animals, the expansion of this subset of monocytes was suppressed and there was a significant decrease in viral replication in this subset.
These data suggested to us that the neuroprotective effect of antiretroviral therapy was mediated by the suppression of this particular subset of monocytes that were no longer activated, and were no longer trafficking into the brain. But the reversal of neuronal dysfunction as measured by MRS happened very rapidly, forcing the conclusion that the processes involved were highly dynamic in at least two ways. First, the data are certainly consistent with the creation of the encephalitis due to trafficking of infected monocytes, and that this process is rapid. The equally rapid reversal of these events in the brain suggested that the trafficking may be bidirectional. Either the infected monocytes are rapidly trafficking out of the CNS or they are rapidly dying or are being deactivated in the brain. Second, these data indicate that the neuronal dysfunction that we observe is reversible by endogenous mechanisms because the drugs that we employed do not cross the blood–brain barrier. Moreover, the endogenous neuroprotective mechanisms are robust because we clearly observe reversal of neuronal injury 2 weeks after the start of treatment. We presume that there is a persistence of infected, activated macrophages in the brain at this time, so the endogenous mechanisms that are operating in the brain must be able to compensate and overcome the deleterious effects of these infected macrophages.
In conclusion, these studies using noninvasive neuroimaging of an accelerated primate NeuroAIDS model has led us to hypothesize that with SIV infection there is a rapid turnover of macrophages in the brain that occurs in a time frame of days to weeks, that there are endogenous mechanisms to reverse the neural injury that are highly robust, and that there needs to be a threshold level of macrophages for neuronal injury to be observable. If verified, these hypotheses suggest several possibilities for the treatment of NeuroAIDS. It may be possible to treat NeuroAIDS with only modest reductions in plasma viral loads, by slowing the trafficking of monocytes, or by enhancing endogenous neuroprotective mechanisms.
It would be interesting to try to understand at what stages of this complicated pathogenic cascade drugs of abuse have their effect. Is it at the level of microglial activation, monocytes trafficking, or by hindering endogenous neuronal protection mechanisms? This is a complicated area of research, but it is amenable to study using the animal model I have described today. There may be specific steps in the pathogenic cascade that may be more susceptible to therapy than others, and it may be possible to reverse some of these changes.
L. Chang
Gil Gonzalez just gave this wonderful detailed explanation about MR spectroscopy and what we are looking at with the different chemical peaks. He showed you MRS data from the SIV models; work in our group as well as Gil’s group and many others have also applied this technique to study the living human brain. Therefore, we can monitor brain changes and try to track the brain’s responses to injury due to HIV infection. We had shown many years ago that with increasing HIV disease severity, progression of dementia from mild to severe, the NAA level decreases (Chang et al. 1999a, 2002). In contrast, the chemicals that are indicative of changes in glia, such as myoinositol, and choline compounds, which includes all the water-soluble cholines, and total creatine, are all elevated (Chang et al. 1999a, 2002). Choline compounds and total creatine are three times higher in astrocytes than in neurons even though they are in both neurons and glia (Brand et al. 1993). During early stages of brain injury, we observed glial activation, as indicated by the elevation of these glial markers. As disease progresses, we observed decreases in NAA, indicative of neuronal injury. We have replicated these changes in several different subject cohorts. One study involved a cohort of patients who were naïve for antiretroviral medication because it was unclear how much of the metabolite changes were due to partial treatment affects or due to possible neurotoxic effects from the medications. In the medication-naïve patients, those who had lower CD4 cell counts had high levels of myoinositol and those with higher plasma viral load had higher levels of myoinositol (Chang et al. 2002). It seems that myoinositol is a very sensitive marker that correlates with disease severity and with performance on cognitive tests, such as the Stroop test (Chang et al. 2002). Patients with higher myoinositol levels performed slower on these tasks. Therefore, we have this very sensitive marker and we further showed that this initially elevated myoinositol and choline will decrease (normalize) at some point after treatment (Chang et al. 1999b). We did a more careful study that followed individuals before and every 3 months after treatment, and we found that it takes between 6 and 9 months before you see the normalization of these initially elevated metabolite levels. We then evaluated whether these MRS metabolites are related to some of the inflammatory markers that are expressed by macrophages or glia.
Therefore, we specifically evaluated the relationship between MRS markers and the macrophage chemoattractant protein (MCP-1) (Chang et al. 2004). We also measured CD4 cell counts, plasma and CSF viral load, and MCP-1 before and after 3 months of antiretroviral treatment. We found that as the CD4 count improved and the plasma and CSF viral load both decreased after HAART, the MCP-1 in both the plasma and CSF also improved significantly. What is really interesting is that when we evaluated all of the brain metabolites, using principal component analysis, the MRS metabolites were naturally segregated into a component that included the metabolites that were primarily present in neurons (the neuronal component) from those that were primarily in glia (glial component). We found that the neuronal component correlated much better with CSF MCP-1 at baseline before treatment, and the correlation was less strong after treatment. This suggests to us that the higher levels of MCP-1 in the CSF may have led to lower NAA, which also suggests that perhaps the inflammation has a harmful effect to the neurons.
On the other hand, the glial component—which includes choline and myoinositol in all regions, and creatine in some regions—did not correlate with either serum or CSF MCP-1 at baseline, but it did correlate very well with CSF MCP-1 after treatment (Chang et al. 2004). We hypothesized that before treatment, we had peripheral sources of MCP-1 (in the serum) as well as brain sources of MCP-1 (parallel to that in the CSF). Kathy Conant, while working with Eugene Major, demonstrated that MCP-1 placed into astroglial culture stimulated the glial expression of more MCP-1 (Conant et al. 1998). After treatment, since the serum level of MCP-1 decreased significantly, we are primarily looking at only brain-derived sources of MCP-1 (from monocytes that migrated to the brain and from glia), and that is why we saw a better correlation with the CSF MCP-1. This study illustrates that we could try to assess the immune system or how the immune response is affecting some of the brain imaging measures.
Another imaging technique that we could use to look at brain inflammation is diffusion-weighted imaging. This is a technique that measures microscopic motion of water molecules in the brain. It can be used to assess whether there is increased water content or decreased tissue with cell loss, which would be reflected in increased apparent diffusion coefficient or decreased fractional anisotropy. In all of the brain regions that we measured, we observed higher diffusion of water molecules in the HIV patients compared to controls, with significant group differences in the frontal white matter (Cloak et al. 2004). We showed that this frontal white matter apparent diffusion coefficient had an inverse correlation with cognitive performance so that the individuals that had higher diffusion had poorer performance on the cognitive tests. Those were also the patients that had higher myoinositol levels, which reflected greater glial activation. These data suggest that brain inflammation (with higher diffusion and myoinositol levels) is associated with poorer cognitive performance. Again, these are different ways to try and measure changes in brain inflammation and looking at whether there is inflammation going on in the brain.
We have also been interested in looking at the interaction effects between HIV and methamphetamine. As some of you discussed earlier, it is really difficult when you have patients who are using multiple drugs, and with imaging studies being so complicated and expensive, we have to be selective with the “drug of choice” used by our research participants. In the past I have focused primarily on HIV or primarily on METH. Our recent studies evaluated potential interaction effects and addressed whether the injury is due to additive, synergistic, or interactive effects, between HIV and METH. Using MR spectroscopy, we studied a group of patients from the Los Angeles area; and as mentioned by Eliezer Masliah earlier, we were able to find individuals who primarily abused METH and nothing or very little else, and they used 0.5–3 g/day, every day for many years. We studied four groups of individuals, HIV only, METH only, both or neither, and we were able to evaluate whether there were additive effects in those with comorbid conditions. We indeed observed additive effects in all three brain regions that we measured (the frontal white matter, the frontal gray matter, and basal ganglia). We observed the greatest decreased neuronal marker NAA (−9%) in the striatum or the basal ganglia region in those with combined conditions (Chang et al. 2005). Choline compounds and myoinositol, as I discussed earlier, are glial markers, and we also observed an additive effect primarily in the frontal white matter in HIV subjects who abused METH (Chang et al. 2005).
More recently, we became interested in studying another drug that is commonly abused by HIV patients, marijuana. I just moved to Hawaii recently, and I would say that up to 80% of our HIV-positive research participants are using marijuana at various levels. We got really interested in learning whether marijuana might also interact with HIV on brain metabolites. This study was done at Brookhaven National Laboratories on a high field (4 T) MR scanner. Marijuana is thought to have immunosuppressive effects. In this particular study, we were looking at glutamate levels and we saw that the glutamate levels were decreased in both marijuana users and HIV but the level seems to normalize in HIV patients who were using marijuana (Chang et al. 2006). The drug seems to have a protective effect, but this effect was seen only in the frontal white matter. In contrast, in the basal ganglia, we actually observed decreased glutamate with marijuana use. Therefore, these metabolite changes associated with drug use are very region-dependent as well (Chang et al. 2006).
I will now present data from recent PET studies we conducted to measure dopamine functions in HIV patients. I was very fortunate to have worked at Brookhaven and collaborated with Dr. Volkow and a group of outstanding radiochemists who were able to synthesize tracers, such as C-11 cocaine, which binds to dopamine transporters (DAT) on the presynaptic dopaminergic terminals, and C-11 Raclopride, which binds to the D2 receptors postsynaptically. Using these same techniques, we also studied a group of methamphetamine users that we transported from Los Angeles to New York for their PET scans, and we found that methamphetamine users had decreased DAT, which correlated with poorer memory (recalled fewer words) and motor function (timed gait) (Volkow et al. 2001b). They also had lower D2 receptors (Volkow et al. 2001a). When I moved to Brookhaven a few years later, one of the first projects that I initiated was to study HIV patients because clinically we observed psychomotor slowing and signs of Parkinsonism in late stages of AIDS dementia. We indeed observed decreased DAT in patients with HIV dementia and this also inversely correlated with the plasma viral load (Wang et al. 2004); higher viral load was associated with lower DAT. With the limited sample size, the group difference was significant only in patients that had dementia but not in the patients who were neuroasymptomatic. We additionally evaluated the combined effects of HIV and drug use. In the New York area, it was very difficult to find individuals who use methamphetamine only. Therefore, we recruited a group of individuals who abused psychostimulants. The majority of these participants used primarily cocaine, but they also experimented with ecstasy (MDMA) or methamphetamine occasionally. This recent study showed that HIV-positive individuals who abused psychostimulants had even lower DAT and lower D2 receptors than HIV-positive subjects without a history of drug abuse. These findings suggest that HIV may lead to decreased dopaminergic function, which may be exacerbated further by psychostimulant abuse. Therefore, dopaminergic agents (especially agonists) may have a role in the treatment of HIV-associated brain injury.
In summary, these data from our imaging study show that we could observe inflammatory changes in patients with HIV infection. These changes are reflected in the elevated glial markers that correlated with CSF MCP-1, and increased diffusion of water in the brain using diffusion tensor imaging. I did not have time to show the functional MRI data but we also observed increased fMRI signals (brain activation) that correlated with glial markers (Ernst et al. 2003). This correlation suggested that inflammatory changes in the brain might lead to less efficient brain function. In addition to the inflammatory changes, we also documented degenerative changes by using various imaging techniques. By correlating the behavior and clinical immune markers with imaging measures, we have a much better understanding of the disease processes. More work is needed and many more imaging studies can be done to further evaluate the mechanisms of brain injury and interactive effects with drug use and HIV. We need to continue to evaluate and understand the mechanisms of brain injury, so we can develop appropriate treatments; examples of the treatments may include anti-inflammatory agents, dopamine agonists, or even antioxidants. We could use these techniques to monitor treatment effects and we can also use these techniques to assess the effects of HIV and/or drugs on brain development and on brain aging. We could perform longitudinal follow up-studies and also to correlate with genotypes to determine whether the different viral strains or different host genotypes may lead to different responses to treatments. Such approaches may help to direct individualized treatments in the future.
Discussion following session 3
Y. Persidsky
How would you perceive this infiltration of CD3 cells in dorsal root ganglia? Why are they migrating there?
C. Power
This is an excellent question. I guess that it is a response to injury. It makes sense that CD8-positive cells, which appear to be the effective cells in terms of neuronal injury, are probably there as part of the adaptive immune response targeting the virus. We also, interestingly, see it in the brains of FIV-infected cats as well. I should say that there is growing literature on CD8-mediated neuronal injury, not just in HIV but in multiple sclerosis.
Y. Persidsky
So let’s say virus will be effectively suppressed, there will be fewer macrophages and most probably few CD3 cells. Did you or any neurologist in the audience see any reversal by neuropathy by antiretroviral treatment, or is this some kind of epiphenomenon?
C. Power
To some extent, we do see improvement clinically in people who have HIV distal sensory polyneuropathy with therapy. Of course, the complicating factor is that patients who are getting dideoxy drugs fully develop neuropathy and as I alluded to, and this came as a surprise. It looks like some of protease inhibitors might also contribute to neuropathy. Notably, these are indinavir, sequinavir, and ritonavir that we found epidemiologically to be associated with neuropathy.
J.S. Hong
I have a question for Dr. Power. It was a very interesting talk. For this DRG infection and also the patient you see the sensory neuropathy and being not a physician, I really know very little about pain. The question is, in the terms of the mechanisms why people feel pain in the foot, is it because the loss of the nerve terminal or maybe there is some inflammation going on.
C. Power
There is no question that there is inflammation within the nerve and also as Dr. Justin McArthur described, it is a dying back neuropathy. It is likely a combination of multiple effects—inflammation within the nerve, the axon being damaged, and also it looks like the small-diameter neuronal cell bodies within the DRG (i.e., the C and A-delta fibers) are more vulnerable to injury.
J.S. Hong
You mentioned mementine and other drugs and also anticonvulsant plus calcium channel blockers. It could be that they both share one of the anti-inflammatory components, where we used to explain the effects by calcium channel blockade or maybe GABA activation. I found it very interesting.
B. Cox
The neuropathy produced by the HIV drugs sounds to be very similar to the neuropathy seen in certain cancer chemotherapy patients with drugs such as vincristine and so on. Again, there is loss presumably of transported materials to the nerve endings. Those kinds of neuropathies don’t respond very well to opiate drugs or to many other drugs. Part of the reasons seem to be remodeling going on in the dorsal spinal cord affecting sensory perception in general and the ability of opiates to regulate them. Is there a loss of responsiveness to the analgesic affects of opiates in the HIV patients as well?
C. Power
My impression is that, yes, they don’t respond terribly well to the opiates, so it has to be a combined approach with the anticonvulsants plus the opiates. You also raise another issue that Dr. Hong alluded to, remodeling the spinal cord. No one has any idea about this topic. That is really an interesting question that probably needs to be addressed.
B. Cox
Clearly, it is a major area of research in terms of neuropathic pain in general.
W. Royal
My question is for Dr. Masliah. Apparently, some basal ganglia interneurons express opioid receptors, and granted you are working with methamphetamine, but have you seen any changes in those populations, any changes in opioid receptor expression?
E. Masliah
We haven’t looked at opiate receptors specifically, but we have looked at some other receptors on this particular set of interneurons, and one interesting thing is that they do have TNF receptors, and I wonder to what extent they might be mediating some of the neurotoxic effects as it was described in the first part of the meeting this morning. Yes, I was kind of surprised to see that. Actually, I don’t know if you have seen this sort of TNF receptor in other neuronal populations and models.
J.S. Hong
Since most of the work of imaging has been done on the patient or primate, with the technical advancement, how small an animal actually can you image now?
L. Chang
There are different machines for animal imaging as compared to those for humans. We do ours in the clinical system in human scanners but there are higher magnetic field scanners (up to 14 T) with small openings that you can scan mice and rats easily.
A. Nath
I have a comment regarding the T cell infiltration and T cells in close vicinity of neurons. It reminds me of a paper that was published by Diane Langford and Eliezer Masliah showing T cell infiltration into the brain of HIV-infected individuals. We have observed the same thing in HAART-treated patients. Carol Petito showed me some data when I visited her very recently, that in post-HAART era, she is finding significant T cell infiltrates in the brain, particularly sitting in close vicinity of neurons. That, to us, is a very grave concern, because we think this may represent a type of immune reconstitution syndrome. And that the mechanism of neural injury might be very different from what we have been thinking of all this while, i.e., all neuronal injury is macrophage-mediated in HIV-infected patients. We may have to rethink the pathophysiology of HIV dementia in the post-HAART era and consider new modes of treatment. So I was wondering what your thoughts might be on this possibility?
E. Masliah
Interestingly, at least in the human cases, we don’t see that much perineuronal or neuronal associated infiltration, although I wouldn’t be surprised that is indeed the case in certain regions, but most of the infiltration that we see is perivascular or clear vasculitis type of injury.
C. Power
I have a question for Linda. Do you tailor your neuropsychological test battery specifically to people who are drug abusers versus those who aren’t? It seems that you have a lot of complex interactions there. I would imagine you’ve probably given this a lot of thought as it is relevant for clinical trials.
L. Chang
We tried to expand the initial neuropsychological test battery that we were using in HIV patients to include additional tests that had been shown to be sensitive for detecting cognitive deficits in drug users. We searched the literature to figure out what some of the reported deficits in drug abusers are and what’s reported for methamphetamine and cocaine, which might be different from the other drugs of abuse too. Because HIV and the psychostimulants are the conditions that I was interested in, and both happen to affect primarily the dopaminergic system, there is actually a lot of overlap in the cognitive batteries.
Session 4: Virus–drug and immune–drug interactions
T. Rogers
Let me begin by pointing out that this next session deals with some of the implications of the direct interaction of drugs of abuse with the immune system. Many of the questions that have been posed in this body of the literature are questions that have been addressed primarily in vitro, and so some of the physiological implications of these studies still have to be addressed.
Work that we and others have reported has already been reviewed to some degree today at this meeting (Rogers and Peterson 2003). Opioid receptors, when activated, are capable of regulating an immune response in a variety of ways, including to regulate levels of chemokines and chemokine receptors. For example, the activation of the mu opioid receptor with agents such as morphine leads to an up-regulation of chemokines such as MCP-1, RANTES, and IP-10. Our work, and the work of Madhavan Nair (SUNY) and Phil Peterson (U. Minn), showed that this up-regulation can be observed in both uninfected and HIV-infected cells, in the periphery as well as in microglial cells and astrocytes in the CNS (Mahajan et al. 2002; Peterson et al. 1990; Wetzel et al. 2000). Our work has shown that there is a mu opioid-induced increase in the expression of both CCR5 and CXCR4 by monocytes and activated T cells. This increase in expression of CCR5 and CXCR4 matches up very well with an increase in susceptibility to infection with both R5 and X4 strains of HIV (Steele et al. 2002).
Morphine, as many of us know, is not strictly or selectively a mu opioid receptor agonist. It actually is an agonist for both the mu and kappa opioid receptor, although most of its effects are predominantly mu opioid receptor-mediated. Curiously, the effects of the kappa opioid receptor (or kappa agonists) are quite a bit different from the mu agonists. For example, in microglial cells and in peripheral blood monocytes, kappa receptor activation results in decreased expression of MCP-1, RANTES, and IP-10, and a down-regulation of CCR5 and CXCR4. Specifically, we have found that the expression of both CCR5 and CXCR4 on CD3-positive peripheral blood T cells was decreased significantly at nanomolar concentrations of U50,488H, an alkaloid kappa-selective synthetic agonist that we use in these experiments. Susceptibility to HIV infection with R5 and X4 strains of HIV was decreased to about the same degree. When one evaluates the effects of drugs such as heroin and morphine, which are, of course, relevant for the drug abusing population, what one is actually visualizing are the combined effects of both mu and kappa opioid receptor activation, and they are not always entirely straightforward.
We are studying cross-talk between GPCRs. The regulation that we study is very rapid and occurs at the level of protein function, rather than at the level of gene expression. We spend a lot of our time looking at the regulation of CCR5 and CXCR4 by opioid receptors. The phenomenon that we primarily focus on is known as heterologous desensitization, shown in the next slide. This is a phenomenon that takes place when one GPCR is activated and signals second (unrelated) GPCR in a way that leads to desensitization or inactivation. This is a selective process, in that not all GPCRs are equally susceptible to this type of signaling. In the cartoon, you see a third GPCR that is left totally unaltered in this process. You can imagine that this first GPCR is the mu opioid receptor, and the second receptor is CCR5, and what I will tell you is that there is desensitization of CCR5 when the mu, delta, or kappa opioid receptors are activated. In contrast, the mu opioid receptor is unable to cross-desensitize a third GPCR (e.g., CXCR4 is unaltered).
This next slide shows evidence for the selectivity of this cross-desensitization process. In this case, the assay is a chemotaxis assay that is mediated by either CXCR4 or CCR5 and the experiment is done with primary human peripheral blood monocytes. In these data, the monocytes are initially activated via the delta opioid receptor (although the mu receptor works just as well), and these delta-activated monocytes fail to chemotax toward a CCR5 agonist. In contrast, the delta (or mu) preactivated monocytes manifest an entirely normal CXCR4 chemotaxis response. The kappa opioid receptor-activated monocytes fail to manifest a response to either CCR5 or CXCR4. This is because the kappa opioid receptor is a much stronger cross-desensitizer than are either the delta or mu opioid receptors. The implications of this for HIV susceptibility are shown here. In this case, what we have done is preactivated the mu opioid receptor with a synthetic, highly selective mu agonist, termed DAMGO. After activation of this receptor, we subject PBMCs to infection with either R5 or X4 strains of HIV. Two hours after infection, we examine an early event in HIV replication, in this case the reverse transcription of the HIV-LTR. The data show Southern blots of quantitative PCR for the HIV-LTR. Basically, the results show that mu opioid receptor activation leads to an inability of CCR5 to function as an HIV coreceptor. In contrast, since the mu receptor is unable to cross-desensitize CXCR4, the coreceptor function of CXCR4 remains intact (Szabo et al. 2003).
We believe that while this phenomenon is taking place in vitro, it is taking place at the same time when the cells are also having their CCR5 and CXCR4 receptors up-regulated, so we believe this is a part of the complex effects that opioids have on cells of the immune system. So there is an initial stage of the mu opioid effect where this cross-talk between the GPCRs takes place. During this early period, susceptible cells become less susceptible to infection with R5 strains of HIV. This is followed after 24–48 h by a shift where the cells become much more susceptible to infection because of the increase in CCR5 and CXCR4 gene expression (Steele et al., 2003). In this late stage, cells become more susceptible to infection with HIV. This late stage effect is likely to be of a much longer duration. The overall impact of agents such as morphine, and other mu opioid receptor agonists, represents this complexity of positive and negative effects (Fig. 9).
Diverse effects of mu opioids on susceptibility to HIV infection. Mu opioids induce a rapid desensitization of CCR5 (but not CXCR4), which coincides with loss of CCR5 coreceptor function, and reduced susceptibility to HIV-1 infection by R5 strains. This early phase is followed by 24–48 h with increased expression of CCR5 and CXCR4 on both T cells and monocytes. This causes increased susceptibility to infection by both R5 and X4 strains of HIV-1.
To sum up, the final slide shows possible topics for additional discussion on the effects of drugs of abuse on HIV susceptibility. There is disagreement between some of the epidemiology and much of the cell biology that has been reported in the literature. I think much of the apparent disagreement here is due to the fact that the effects on the immune system are not so straightforward. In my view, careful examination of the literature actually shows that studies on the cell biology point to a mixed effect of opioid drugs of abuse on HIV susceptibility. At the same time, careful examination of the epidemiology suggests some effect on the progression of HIV infection and disease, but both the cell biology and the epidemiology are incomplete. Perhaps, the most important point here is that we have not asked the most important questions about the impact of drugs of abuse on HIV susceptibility and progression to AIDS. In my view, it is time to carry the studies forward and ask the more difficult questions.
There is also an impact of drugs of abuse on HIVE, and there is epidemiology that supports this. Here again, there is complexity both in terms of the inflammation and HIV replication. Finally, when one considers all of this, it is important to keep in mind that there are efforts being made to develop novel antiviral compounds. For example, CCR5 antagonists are being developed and are currently in clinical trials, where there is both an inhibition of HIV infection, coupled with an impact on a component of the acquired immune system. It isn’t so clear at this point whether these types of therapeutics might pose problems for the intravenous drug abuse population where the immune system is compromised.
W. Ho
I have an ongoing study investigating interaction of opioids, substance P, and HIV in the immune system. I am very happy some of you mentioned substance P in an earlier session of this workshop. I hope I can talk about this topic later. The reason I made a decision to talk about drug abuse, HIV, and CNS innate immunity for today’s presentation is because I feel this is an underdeveloped area, and possibly this is a direction for future research. There is little information about the impact of opioids on CNS innate immunity although the immunosuppressive effects of opioids have been studied extensively (Eisenstein et al. 1993; Nair et al. 1997; Peterson et al. 1987; Stoll-Keller et al. 1997). I meant intraneuronal immunity. I am not talking about the macrophage- or T-cell-mediated innate immunity. I am going to talk about neuronal cell-mediated innate immunity. We all know about the innate immunity, so one of the important components in the host innate immunity is IFN-α (Pestka et al. 1987), which was discovered over 50 years ago. Although we know much about IFN-α, the information about the impact of opioids on IFN-α-mediated innate immunity is lacking. Several lines of evidence show that IFN-α actually binds to opioid receptors in the CNS, and interacts with opioids (Dafny 1998; Dafny and Yang 2005; Wang et al. 2004). Neurons produce type I interferon during viral infection in the CNS (Delhaye et al. 2006).
IFN-α inhibits viruses, HIV, hepatitis C virus (HCV), and other viral infections. In terms of HIV infection, we know IFN-α is an important inhibitor of HIV infection of macrophages. Therefore, we examined IFN expression in the CNS cells. The first thing we did was to see if the neuronal cells express type I IFNs. We know the cells from the immune system express IFNs. NT2-N cells, human neuronal cell line express IFN-α. CHP212 cells, also a neuronal cell line, express IFN. We also looked at the other elements in the IFN pathway in the neurons. As you can see, there are regulatory factors, STAT1, IRF3, IRF5, and IRF7, which are the most important factors in the activation of IFN pathways. We see IFN expression in both the immune cells and the neuronal cells. The most important question we would like to ask is whether morphine and/or HIV inhibit IFNs and related cellular factors in the neuronal cells. In both cell lines (NT2-N and SY5Y), the morphine significantly inhibited IFN-α expression in a dose-dependent manner. In this particular neuroblastoma cell line (SY5Y), you see there is a dramatic decrease in IFN-α expression by morphine. This kind of effect is exciting, because we all want to see 5- or 10-fold differences.
Well, let’s see whether HIV proteins (gp120 and tat) have effects on endogenous IFN-α expression. As demonstrated in this slide, there is little effect of the HIV proteins on IFN-α expression. However, we do see some negative effect of HIV Bal infection on IFN-α expression in the human neuronal cells. HIV Bal is from cultured macrophages. In any case, we have to study mechanisms involved in the impact of morphine and/or HIV or HIV proteins (gp120 and tat) on IFN-mediated innate immunity in the neuronal cells.
What I am interested in is to understand how morphine and/or HIV interfere with IFN pathways. As I mentioned earlier, there are several important IFN regulatory factors (IRF3, 5, and 7) that have key roles in the activation of IFNs. I have some data published in the journal Hepatology, demonstrating that morphine, through the inhibition of IRF5 and IRF7 expression, blocks interferon expression in human hepatocytes (Zhang et al. 2005). As indicated in this diagram, there is a possibility that morphine also affects the translocation of IRFs, which suppresses interferon expression. Morphine may interfere with the binding process of endogenous IFNs, which is what we want to study in the near future. We know that in order to exert biological activities, endogenous IFNs need to bind to their own receptors on the cell membrane (Aguet and Mogensen 1983; Langer et al. 1996). Thus, factors that block IFN binding to their receptors are critical in suppressing IFN-mediated innate immunity. It would be of importance to examine whether opioids and/or HIV function are factors in terms of the inhibition of IFN pathway. We do not know whether morphine or HIV use the same mechanisms(s) to compromise IFN-mediated innate immunity in the neuronal cells. In summary, in order to examine the interactions, the impact of opioids and/or the HIV proteins on intracellular IFN-mediated innate immunity in the neurons will contribute not only to our basic understanding of host cell innate immunity against HIV, but also to the design and development of innate immunity-based therapy and prevention strategies for HIV-infected opioid abusers.
W. Royal
There was an observation over a decade ago that vitamin A deficiency caused HIV-infected individuals to develop HIV-related complications. Pregnant women who were vitamin A-deficient were more likely to transmit virus to their newborns and HIV-infected drug users who were vitamin A-deficient were more likely to have lower CD4 and to progress more rapidly to AIDS. Since that time, a number of studies have been done that looked at the potential benefits of vitamin A supplementation in HIV infection. The results have been mixed. This may be due to the fact that when we are talking about Vitamin A, we are really referring to a group of different, although related, compounds. In blood, the predominant form of vitamin A is all-trans retinol. All-trans retinol is converted to all-trans retinoic acid, which is further converted to 9-cis retinoic acid and 13-cis retinoid acid. 13-cis Retinoic acid is the drug Accutane. These agents act by binding specific retinoid receptors. There are two families of retinoid receptors: retinoid acid receptor, which is also referred to as RAR, and retinoid X receptor, which is referred to as RXR. For each receptor family, there are alpha, beta, and gamma subtypes, and the mRNA for these receptors can be transcribed from either of two promoters. RXR can either exist as a homodimer or form a heterodimer with RAR. Therefore, you can imagine that, given the different subtypes and variants for each, one can potentially find a large diversity of receptor dimers that can be formed.
The retinoid receptors can dimerize not only with each other, but also with other receptors, including the peroxisome proliferator activator receptors, the vitamin D receptor, and the thyroid hormone receptor. The key player in these interactions is RXR, which turns out to be a partner for all of these. The outcome of such dimerization can be either activation or suppression of the specific gene transcriptional activity. This occurs because these receptors act as transcription factors that can interact with members of the transcriptional complex and can directly bind to response elements in the promoter regions of genes so that one can get up-regulation or suppression of gene expression.
We looked at the issue of a possible interaction between the retinoid and opioids, keeping in mind the clinical observation made in drug users who were vitamin A-deficient, and developed an in vitro model system in which to examine it. We used U937 cells, which is a human monocytic cell line which, following activation with phytohemagglutinin (PHA), will express TNF-α. We examined both TNF-α secretion by activated cells and also performed flow cytometry and looked at intracytoplasmic TNF-α expression and the percentage of cells that were positive for this cytokine. When we activated these cells in the absence of opioids, we found that we could increase TNF-α expression with PHA activation, and we were able to suppress that activation with all of the retinoids that we used. What we used in these studies was 9-cis retinoic acid, which binds RAR and RXR, all-trans-retinoic acid, which binds RAR, a synthetic RXR-selective agonist (LG101305), and antagonists for RAR and RXR (LG100815 and LG101208, respectively). Interestingly enough, when we included both opioid and morphine in these experiments, we found that wherever there was RXR activation taking place, induced by either directly activating RXR with 9-cis retinoid acid or LG101305 or by antagonizing RAR, the suppression of TNF-α was inhibited. In contrast, if we activated retinoic acid receptor or blocked retinoid X receptor, we did not see that morphine affect. That was observed with examining both TNF-α-positive cells by flow cytometry and secretion of TNF-α in culture supernatants.
Since we were using morphine in our studies and seeing these effects, we decided to look to see whether opioid receptor expression itself was somehow involved. We did that by examining mu opioid receptor expression using flow cytometry. For these studies, we used an antibody that binds to the N-terminal of the protein, which allowed us to use intact cells in these experiments to examine surface expression. What we observed was similar to the data obtained in our studies on TNF-α expression, i.e., activating the cells in the context of RXR agonists or RAR antagonists increased surface mu opioid receptor expression, whereas an opposite effect was observed when the cells were exposed to RAR agonists or RXR antagonists. We also looked to see if we were just not getting changes in mu opioid receptor expression but also associated alterations in opioid receptor binding. We did that by labeling our treated cultures with fluorescein naloxone and analyzing the intact cells by flow cytometry. Again, with activating the cells we saw an increase in opioid receptor binding on the surface of the cells and this binding was detected by flow cytometry. The binding persisted if we treated the cells with either the bifunctional agonist (9-cis retinoic acid) or the selective RXR agonist (LG101305) or if we antagonized RAR with LG100815. If we bound RAR or antagonized RXR, however, surface opioid receptor binding decreased. If the cultures were also exposed to morphine, no opioid receptor binding was detected, demonstrating that the retinoid–opioid receptor interaction that is observed in our system is receptor-specific.
We did further studies to determine whether this effect might be observed at the promoter level, since it is known that the retinoid receptors are essentially transcription factors that become part of the transcriptional complex to affect gene expression. To do this, we used a plasmid construct composed of a human mu-opioid receptor promoter linked to a luciferase reporter and transfected the same U937 cells and activated the cells in the presence and absence of the retinoids and morphine or both. In the context of exposing the cells to RXR agonists or RAR antagonist, we saw increased opioid receptor promoter activation that was enhanced by the presence of morphine. In contrast, promoter activation was blocked by antagonizing RXR and with RAR agonist.
These studies were performed in an in vitro system. We now have the opportunity to look a these issues in a well-characterized in vivo system, the HIV transgenic rat that was developed by Joe Bryant at the Institute for Human Virology at the University of Maryland. These animals develop many of the manifestations of HIV disease, including neurological disease. We feel that the transgenic rat model will be quite useful for studying interactions between opioids and retinoids. Since this model replicates so many different aspects of clinical HIV disease, we are certainly interested in retinoid and opioid effects on currently well described manifestations and would be interested in ideas as to what other clinical markers would be useful to follow as we do these assessments.
Y. Persidsky
My laboratory is very interested in the structural and functional impairment of the blood–brain barrier and how it affects leukocyte trafficking into the brain. We also study drug penetration and how the blood–brain barrier could exclude penetration of toxic compounds/factors. The role of peripheral immune responses in control of HIV-1 encephalitis and in the pathogenesis of neurodegenerative disorders are research areas that are rapidly emerging. A number of speakers have pointed to this already. Another critical issue is how antiretroviral drugs penetrate the blood–brain barrier and what we can do to change their entry by modifying the barrier’s permeability. Parallel questions for how inflammatory responses are modified by HIV proteins and influence permeability are also important areas of research. This definitely applies to drugs of abuse and also the potential comorbidity factors in disease.
With regards to immunopathology of the blood–brain barrier and brain in HIVE, I am pleased to review this with you. Here are examples derived from more than 30 brains: control without HIVE and HIVE at increasing stages of disease pathology. Staining is for tight junction protein, claudin 5, one of the major proteins assuring “tightness” of the blood–brain barrier. It is double stained with CD163, considered to be one of the more specific markers of perivascular macrophages. In the control brain, there is obvious staining of all the microvessels including individual capillaries (Persidsky et al. 2006a). In HIVE, we have infiltration of CD163 positive monocytes/macrophages, which—according to the studies in monkey models of NeuroAIDS and in human peripheral blood—plays an important role in disease. There is a significant decrease in staining of the tight junction protein. A correlation exists between the level of macrophage infiltration and disruption of the blood–brain barrier. We had an opportunity to use primary brain microvascular endothelial cells from human brains at low passages. We mimicked the blood–brain barrier by using the transwell system. Coculture of endothelial cells and infected or uninfected monocytes, which actually migrate across the blood–brain barrier, led to significant up-regulation of activity of GTPases, Rho, which controls cytoskeleton. We were able to demonstrate that if we blocked activation of Rho by applying Rho-specific inhibitors in our transwell system, we can inhibit migration of monocytes across the blood–brain barrier (Persidsky et al. 2006b). It was also blocked at the level of endothelial cells or monocytes. Migration in this particular situation was in response to CCL2 (MCP-1), which is the major chemoattractant in HIVE.
We further expanded our studies by examining functional changes in tight junction proteins and in examining their phosphorylation as it would affect increased permeability of the barrier and leukocyte trafficking. Interactions between primary brain endothelial cells and monocytes resulted in the phosphorylation of occludin and claudin-5, two major tight junction proteins. In some experiments, HIV-1-infected cells actually produced more pronounced phosphorylation. Inhibition of acting downstream of Rho, Rho kinase, also blocked phosphorylation of tight junction proteins and inhibited migration of monocytes across the blood–brain barrier. Such approach may be useful for treatment of HIVE.
Another way to assess blood–brain barrier dysfunction is to look at how transport systems protecting the brain from blood toxins could be altered in HIVE. Here are the same brains but now stained for multidrug resistant associated protein, breast cancer resistant protein (BCRP), or P-glycoprotein (P-gp). In the brain of a noninfected person, there is strong staining for BCRP, while in HIVE using the same markers, BCRP and CD63, there is significant disruption of the BCRP staining paralleling monocyte infiltration (Persidsky et al. 2006a). Importantly, we were able to demonstrate up-regulation of BCRP in microglial modules, signaling that there is decrease in expression on endothelial cells and increase in HIV-infected and immune-activated cells.
Similar studies were performed for P-gp indicating the same trend of down-regulation of P-gp on endothelium and increase in cells of macrophage lineage. We confirmed the studies by using Western blot to detect expression of P-gp and BCRP in protein isolated from the same brain tissues. Why is it important? P-glycoprotein substrates include both morphine as well as HIV protease inhibitors. On the other hand, BCRP, which is as heavily expressed in the blood–brain barrier, is also a transport protein for reverse transcriptase inhibitors and most probably for protease inhibitors. Definitely, there is some interaction between drugs of abuse as well as transport of antiretroviral drugs. BCRP expression in human macrophages was increased after HIV-1 infection and demonstrated by FACS and Western blot staining. These results were also observed following immune stimulation with relevant proinflammatory cytokines, which increased the BCRP levels even further than what was seen with infected cells alone.
In contrast, decreased BCRP on brain microvascular endothelial cells was demonstrated after TNF-α treatment. The studies were extended by performing functional assays assessing accumulation of substrates for specific transporter in the endothelial cells. Proinflammatory cytokines decreased such functional activity leading to increased substrate accumulation. These effects were mimicked further in endothelial cells in situ.
With regards to peripheral immune responses, we studied CD8+ T lymphocytes accumulations in disease and demonstrated that they are specifically distributed in the areas containing HIV-infected macrophages/microglia during HIVE. We assumed that these cells affected neurodegeneration by stimulating neurotoxin production from infected macrophages. Part of these changes might be related to over expression of indoleamine 2,3-dioxygenase (IDO), a key enzyme for tryptophan metabolism and neurotoxicity due to quinolinic acid. IDO could lead to impaired immune responses. Microglia and macrophages express high levels of IDO during HIVE.
Most importantly, interaction between CD8 cells and cells forming microglial nodules could promote suppression of immune responses (Potula et al. 2005). How would this be related to drugs of abuse? Nair and colleagues demonstrated that cocaine up-regulates expression of IDO (Nair et al. 2004). We demonstrated in our in vivo animal model that efficient inhibition of IDO results in enhanced immune responses, doubling the amount of CD8+ T effector cells and more efficient elimination of HIV-infected macrophages. On the other hand, we fully understand that this approach may lead to something similar to immune reconstitution and some kind of neuroprotective strategies will be required.
The blood–brain barrier is an important area to study in terms of drug permeability, interactions with drugs of abuse, potential increase in expression of transport proteins, leukocyte migration and their functional interactions at tight junctions, immunomodulatory effects of drug abuse in the CNS, and antiretroviral responses. It also provides opportunities to use cell-based drug transport system that are being developed by Dr. Howard Gendelman's group in our center (Dou et al. 2006).
H. Fox
Since we have heard a lot about encephalitis and the like so far, I would like to talk about the chronic infection state, what we call the relatively stable phase of disease. This is for a number of reasons: fortunately, people are living longer with the virus, and even in those countries that don’t have therapy, this is the most common stage of the pandemic because once you develop immune deficiency, if you don’t have therapy you don’t do well. As we all know, fortunately the incidence, at least in this country, of neurocognitive disorders has dropped, but with people living longer the prevalence may be increasing.
The brain is an interesting place—it has a unique virus–host interaction, and you get virus in the brain very early after HIV, or else you get reinfection and it stays there. I think, as Monica Carson has brought up with some of our favorite cells, the microglia, their adaptation and the reaction of CNS can be both protective and damaging. Those cells aren’t there to damage the brain, but over the long term, although a viral host interaction is fine for your spleen and lymph node, it is probably not that great for the brain over 10 to 15 years.
What else is unique about the chronic phase, because of the time period, you have a much greater opportunity for interaction with other coexisting diseases. Hepatitis C has been mentioned today, issues such as aging and the subject of this meeting, drugs of abuse.
Fortunately, with animal models (I work on the SIV-infected rhesus macaque model), we can obtain both molecular and mechanistic profiles during these disease stages (Roberts et al. 2003, 2004a, 2006), which allow us to find the basis of these diseases and hopefully the means to intervene in their progression.
We have published a lot on the various stages of disease in the monkeys (Fox et al. 2000; Marcondes et al. 2001; Roberts et al. 2003, 2004a, 2006). One report that is just coming out now is about molecular profiling of what we call the chronic stage (Roberts et al. 2006). These were monkeys that were infected for approximately 2 years, and relatively healthy, other than having SIV on board. They had alterations in their neurophysiology (sensory evoked potentials), but otherwise had normal CNS function. Although in our studies in encephalitis and acute infection, where as expected we have hundreds of genes up-regulated, in this chronic disease stage we have very few. Interferon-mediated genes have come up a bit in the presentations by Wenzhe Ho and Eliezer Masliah, and certainly in the acute infection and in encephalitis we see tons of these interferon-induced genes (Roberts et al. 2003, 2004a). Surprisingly, we can’t find type 1 interferon, which is the main inducer of these genes. The bioinformatics signature of all the genes that are up are certainly the interferon or Stat-1 mediated pathway, but this appears to be an interferon-independent pathway that is initiated by the virus and potentially other factors. So, especially with the methamphetamine findings of Eliezer Masliah and possibly some opioid findings, this Stat-1 pathway of interferon-like induced genes is important not only acutely and at the end stage, but now we find them in the chronic stage, too. G1P3 and the HLA molecules, which we find increased in the chronic stage, can be induced by interferon.
One thing I wanted to focus on here is an example of what we can get out of this. CCL5, which you all know as RANTES, a nice chemoattractant, also has a number of effects both neurophysiologically, in apoptosis, and in differentiated neurons in slice preparations or culture conditions. Compared to controls, RANTES/CCL5 goes up in the acute stage, and drops down in the postacute stage, when the viral load is reaching a steady state in the blood, but it is still significantly elevated over controls. It then rises in what we call the early chronic phase, about 9 months after infection. Two years after infection it continues to rise, and in SIV encephalitis it reaches its highest levels (Roberts et al. 2006).
What cells are making RANTES and what cells respond to RANTES? Another aspect of our work that we studied for a long time are the CD8-positive T cells, which we find infiltrate the brain after infection. Fortunately, in monkeys we can look for it pretty sensitively because we can perfuse the brains, make homogenates, cell suspensions, and look for CD8 T cells. You see here the number of CD8 T cells we can recover per gram of brain tissue rises from 20,000 up to approximately 60,000, a significant increase (Marcondes et al. 2001; Roberts et al. 2006). We get increased numbers of T cells in the brains of these chronically infected animals, with low but measurable levels of virus. What do we see in the brain as opposed to encephalitis? We see variable but few macrophages. This is almost at normal levels here so you can see by the CD163 staining (Roberts et al. 2004b), but occasionally in the perivascular areas you can find groups of macrophages slightly increased over normal. Very rare cells are positive by in situ hybridization for SIV. This one here is perivascular, although it is a little hard to see with the counterstain. This one here is within parenchyma producing SIV but as I say, these are rare. We can detect CCR5/RANTES by using immunohistochemistry. We can find it here on lymphoid-like cells surrounding these two little pale neurons. That is very rare. You do tend to find them within the perivascular region as Eliezer Masliah just described with CD8 T cells. This section is stained for CCL5/RANTES, which is present in the cytoplasmic granules, and the serial section is stained for CD8 to identify these as CD8 T cells, which by and large are cytotoxic T cells. From other work, we think at least half and likely the vast majority of these infiltrating cells are SIV-specific.
Of course, we are here to talk about interactions with drugs of abuse. We have heard about rodent dosing and how difficult it is to really get a good model of chronic dosing, and it is even more difficult in monkeys. What we did is use an increasing dose protocol (Madden et al. 2005). We did this for a number of reasons, first of all to mimic the human condition and second of all because methamphetamine is pretty toxic. Those of you who have worked in emergency rooms know that. Where it can be toxic in humans and especially monkeys is hyperthermia. We found that if one slowly ramps up the dose, they get tolerant to the hyperthermic effect, but not so much to the anorectic effect; actually, they have decreased food intake but are still healthy (Madden et al. 2005). We also measured urinary cortisol. Urinary cortisol is increased, so there is a degree of stress that goes along with this. Because we looked at CCL5/RANTES in the previous study, we wanted to see if it was increased by methamphetamine and indeed in the frontal lobe it was—but it wasn’t increased in the caudate nor in the hippocampus. IL-1, though, was not increased in the frontal lobe or the caudate but was increased in the hippocampus. This continues that theme about region-specific affects of drugs and glial cells and SIV and HIV. We haven’t looked at the interferon-stimulated genes yet. I think that it will be interesting to correlate with the human data.
We did infect methamphetamine-treated monkeys and followed them for 9 months. Our main test for CNS function was neurophysiology and we used our most sensitive measure, brainstem auditory evoked potential. You can see that P5, which is the last wave, becomes abnormal here approximately 3 months after SIV infection. With methamphetamine treatment, it became abnormal earlier, and an additional wave became abnormal. Pathologically, with methamphetamine alone, we didn’t see many activated microglia or macrophages in the brain. We did see some in SIV and SIV + methamphetamine, but there was no statistical difference between the groups. We have not looked at astrocytes or measures of astrocyte activation. There was no encephalitis in any of these groups, but they weren’t long-term studies. Outside of the brain, it was interesting. In the SIV + methamphetamine group, three of the four had SIV-induced lung disease, and three of the four had renal disease. In the SIV only group, one of the four animals had focus of lung disease. Of course, the problem here is the small sample size, three out of four versus one out of four. This leads to our concept of the interaction between drugs and SIV.
My questions would be: in which mechanistic or pathogenic pathways do drugs of abuse and SIV or HIV interact in vivo, because I work a lot in vivo. We can certainly, and I have too, demonstrated a lot of things in culture and whether the relevant in vivo is still the question. The concept is that the brains were fairly equivalent, but with methamphetamine we saw the development of AIDS. Are the effects predominantly peripheral versus the brain? Do you get more immune suppression and thus more rapid HIV related disease and an increased chance to get CNS disease? Are these things (the untoward effects of drugs of abuse on HIV infection) really acting on the periphery, not the brain, and the brain is a secondary effect, although it is one of the prime things we think about and worry about.
Discussion following session 4
A. Nath
Howard, I really enjoyed your presentation. It is interesting that you noticed in cytotoxic T cells in the perivascular region and I remember talking with you about it earlier. I wonder if you immunostained them for granzymes, to know if they are producing granzymes or not.
H. Fox
In an earlier study we did immunohistochemical staining for granzyme B and did see it. We have not stained these brains, and we should because it is actually much more common here and we can tell their localization better. If I can just elaborate just a little, I think the concept of the vasculitis as mentioned earlier is interesting, although you don’t really see vascular damage.
A. Nath
It is perivascular.
H. Fox
But when you think of it in terms, let’s say as Gil Gonzalez said, of infiltrating macrophages, which tend to be perivascular, at this stage of the disease that (perivascular) is where virus is. Even in late stages, there is more virus there than in the parenchyma. Is this a smoldering thing and the perivascular macrophages induce T cells to come in and cross-talk and an interaction gets going there, that either spills into the parenchyma, ruins the blood–brain barrier or who knows what. Long answer, did I answer your question? We will look into this further in upcoming work.
J.S. Hong
Do the T cells that infiltrate the brain replicate?
H. Fox
We haven’t proven that the lymphocytes replicate in the brain. But the tetramer staining allows you to say these are definitely SIV-specific, so it is a complex of MHC class 1 and the SIV peptide that can only bind to the T cells that recognize that specific epitope. We didn’t do BrDU labeling or anything like that, but we can stain with Ki-67, which implies that they are definitely in the cell cycle. I think their accumulation in the brain speaks to that, too.
J.S. Hong
The second question is related to the blood–brain barrier. We understand that opening up the barrier would be very important for the trafficking of therapeutic drugs. In terms of the trafficking of the monocyte or macrophage, do you think it is important to open up the barrier, or is it that some sort of chemoattractant could be also very important? In other words, can the monocytes be trafficked into the brain without affecting the barrier?
Y. Persidsky
I think that what we are seeing in encephalitis most probably is an exaggeration of what we see under normal circumstances when there is normal trafficking of macrophages in the brain and changes in these perivascular brain compartments. At that point, there is probably no significant disruption of the blood–brain barrier; but when you have, for whatever reasons, massive infiltration driven by high expression of chemokines (as well as some other stimuli that we still don’t understand well in CD8-depleted monkeys), there is a compromise of the blood–brain barrier and there is importance of migration. It is quite clear that if you change or affect of blood–brain barrier in vitro and to extend in vivo, you can actually change the pattern of leukocyte and monocyte migration.
C. Power
Two questions for Dr. Royal, the first, does retinoic acid influence infectivity? In other words, could it be modulating infection? The second question is did you see any neurobehavioral effects in the animals that were depleted or were vitamin A deficient?
W. Royal
We haven’t looked at infectivity and neither have other investigators, to my knowledge. But it is clear that the HIV LTR contains retinoid receptor response elements and that retinoids can modulate HIV replication. We would be interested in seeing what happens in vivo with respect to retinoid effects on HIV infectivity as well as examining the neurobehavioral effects of retinoids and opioids in this model.
J. O’Callaghan
I wanted a clarification from Dr. Fox. Did the time point when the microgliosis was observed overlap with the RANTES, IL-6, and IL-1 increase?
H. Fox
There was increase in some markers but no significant difference between the two.
J. O’Callaghan
Did you see this with methamphetamine alone?
H. Fox
No, we did not see it in methamphetamine alone, but let me clarify that microgliosis was characterized by staining with CD163 and HLA-DR. With the immunostaining we did pick some up, but I will add that there are different patterns seen and thus it was difficult to quantify. I was surprised as I thought we would see more.
J.S. Hong
I have a question for Dr. Ho. I was very impressed with one of your slides actually showing the morphine in a very low concentration that affects the expression of interleukin and interferon α. There are two questions: is this effect mediated through opioid receptors? And the second question is whether you tried even lower concentrations, say, lower than 10−12 M?
W. Ho
If you use a high concentration, there will be no in vivo relevance in the CNS. Thus you don’t need too much of these neuropeptides in order to have a biological function. We have data to show that you can block this effect by using naltrexone or naloxone. We used a higher concentration of 10−6 M, and that effect was similar to a lower concentration of 10−10 M. We can go down to 10−14 and still see an effect.
J.S. Hong
Assuming this is really the GPCR-mediated event and we know most of the K d values for GPCR are in the range of nano- to picomolar concentrations. The question is when we see femtomolar concentrations are effective, is this mediated through GPCRs or it is mediated through an entirely different site?
W. Ho
I talked about this particular cell line that gave better results than NT2-N cells in terms of the effect of IFN. But I don’t know why morphine at 10−14 M still had the effect. I guess there is a variation in the experiment using this particular cell line. In any case, I am very confident with the concentration of 10−10 M at this point.
C. Power
I have a question for Dr. Rogers. Is there any evidence that opioid receptors modulate adjunct receptors?
T. Rogers
There is little known at this point about modulation of adjunct receptors, or other proteins which may associate with these GPCRs, following heterologous desensitization. There is evidence in the literature that opioid receptor trafficking typically involves association with additional proteins. Your question is important from the standpoint of the regulation of receptor expression following cross-desensitization. In this case, certain GPCRs are internalized as a part of the cross-desensitization process, and some are not. Why some are internalized is not clear at this time.
Conclusions
Following the talks, representatives from each of the four sessions presented highlights of the presentations in their groups, with a focus on recommendations for future research goals. The opinions expressed are not necessarily those of every investigator, of NIDA, or of the NIH institutes actively engaged in NeuroAIDS research. Originally, these recommendations were to be discussed and prioritized the next day by the entire group, but with nearly 50 distinct ideas presented, it was decided that breakout groups should be formed to identify the top priorities for interdisciplinary research. Four breakout groups were formed from the members of the four presentation sessions. After extensive discussion, each group presented their top four priorities for research related to drug abuse interactions with HIV/NeuroAIDS and/or neuroinflammation, as well as two priorities for “cross-cutting” research that addresses more widespread goals. The highest priority drug abuse-related research questions identified by the participants were as follows:
-
How do drugs of abuse affect blood–brain barrier integrity and cell phenotype, as well as leukocyte trafficking or transmigration?
-
What in vivo models can be used or developed (e.g., small animal imaging) to study the effects of drug exposure on blood–brain barrier transmigration?
-
What are the mechanisms by which drugs of abuse perturb neuronal and glial function, as well as innate brain immunity?
-
How can surrogate markers for NeuroAIDS be defined for humans or animal models, particularly for mild/moderate cognitive–motor disorder in drug abusers?
-
What are the effects of drugs of abuse on cognitive decline and neurodegeneration, using in vivo and in vitro model systems?
-
What are the effects of route of administration, kinetics, and withdrawal of addictive drugs on inflammation and glial responses, specifically systemic versus local CNS responses to pathogens and immune cell infiltration?
-
What are drug targets on nonneuronal cells, including receptors and intracellular signaling pathways in astrocytes, endothelial cells, and microglia?
-
To what extent are glial cells a part of viral reservoir, with respect to persistence, reactivation, release within CNS, and how drugs of abuse affect these reservoirs?
-
How can new models be developed (animal, cell or in vitro) that are practical and faithful to critical drug abuse and HIV/AIDS issues of clinical relevance?
-
What are the effects of acute/chronic exposure to drugs of abuse on immune and neuroendocrine systems especially related to HIV/AIDS, other infectious diseases, and stress?
-
How do drugs of abuse impact viral dynamics, including entry, trafficking, replication, and infectivity?
-
How do drugs of abuse affect pharmacological and behavioral therapeutic outcomes, such as adherence to antiretroviral medications, or self-medication for cognitive or behavioral effects of HIV?
-
What are the imaging and pathological correlates of the white matter abnormalities in HIV/AIDS and/or drug abuse?
-
How do drugs of abuse (including alcohol and marijuana) contribute to peripheral neuropathy in terms of neuropathogenesis and treatment strategies?
-
How does HCV interact with HIV and drug abuse neuropathology?
-
Do specific drugs such as marijuana modulate the immune response to cause neurotoxicity or neuroprotection in the setting of NeuroAIDS and/or other drugs of abuse?
In addition, the following “cross-cutting” priorities were identified:
-
How can specific substances of abuse be matched with models that appropriately mimic and translate to human disease, and how can rapid translation from model to human be improved?
-
How can HIV-dedicated research resources and/or technologies, such as PET or other imaging resources, genomics, and proteomics, be obtained?
-
How can common pathways and targets for neurodegeneration and neuroprotection be defined for multiple pathogenic conditions?
-
How can new technologies be developed (e.g., novel imaging techniques, small molecule therapeutics) for assessment and treatment of NeuroAIDS?
-
How can technology be improved (e.g., novel PET ligands) to measure neurotransmitters in vivo (e.g., GABA, glutamate, glutamine), including in the context of NeuroAIDS and drug abuse?
-
How can genetics, proteomics, lipidomics, and/or metabolomics be utilized to identify biomarkers for susceptibility to neural tissue injury, such as in the context of NeuroAIDS and drug abuse?
-
How can epidemiologic and clinical studies better address the prevalence of NeuroAIDS among HIV-infected patients that use drugs?
-
What is the role of CD8+ and other T cells in controlling virus in CNS compared to the rest of the body, and what are the effects of drugs of abuse on immune reconstitution in the era of HAART?
In conclusion, this interactive panel discussion provides a unique yet broad prospective for the mechanisms of HIV-associated neurological diseases in the context of drug abuse. Particular attention is paid to neuroinflammation, changes in blood–brain barrier permeability, immunity, neurotoxicology, neuroprotection, and glial–neuronal interactions. Alterations in cellular control and regulatory mechanisms may now be developed in the advent of genomic and proteomic technologies applied to NeuroAIDS research (Ciborowski and Gendelman 2006). In particular, development or validation of appropriate in vitro or animal models that allow the concurrent study of HIV or SIV infection and specific patterns of chronic or acute drug exposure would be useful, especially if those models are of clinical relevance. A better understanding of the basic mechanisms of HIV-associated neuropathogenesis and neuronal/glial dysfunction in the context of drug use/abuse is critical for the subsequent development of treatments for neurological complications of AIDS in patients who use drugs.
References
Aguet M, Mogensen KE (1983) Interferon receptors. Interferon 5:1–22
Amemiya K, Traub R, Durham L, Major EO (1992) Adjacent nuclear factor-1 and activator protein binding sites in the enhancer of the neurotropic JC Virus. J Biol Chem 267:14204–14211
Baucum AJ, Rau K, Riddle E, Hanson GR, Fleckenstein AE (2004) Methamphetamine increases dopamine transporter oligomer formation via a dopamine- and hyperthermia-associated mechanism. J Neurosci 24:3436–3443
Benkovic SA, O’Callaghan JP, Miller DB (2004) Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic–clonic seizures. Brain Res 1024:59–76
Block M, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76:77–98
Block ML, Li G, Qin L, Wu X, Pei Z, Wang T, Wilson B, Yang J, Hong JS (2006) Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. FASEB J 20:251–258
Brand A, Richter-Landsberg C, Leibfritz D (1993) Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 15:289–298
Brown JM, Gouty S, Iyer V, Rosenberger J, Cox BM (2006) Differential protection against MPTP or methamphetamine toxicity in dopamine neurons by deletion of ppN/OFQ expression. J Neurochem 98:495–505
Buckner CM, Luers AJ, Calderon TM, Eugenin EA, Berman JW (2006) Neuroimmunity and the blood–brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on NeuroAIDS. J Neuroimmune Pharm 1:160–181
Capitanio JP, Mendoza SP, Lerche NW, Mason WA (1998) Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proc Natl Acad Sci 95:4714–4719
Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix C (2006) CNS immune privilege: hiding in plain sight. Immunol Rev 213:48–65
Chang L, Cloak C, Yakupov R, Ernst T (2006) Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J NeuroImmune Pharmacol 1:65–76
Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E (1999a) Cerebral metabolite abnormalities correlate with clinical severity of HIV-cognitive motor complex. Neurology 52:100–108
Chang L, Ernst T, Leonido-Yee M, Witt M, Speck O, Walot I, Miller EN (1999b) Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology 53:782–789
Chang L, Ernst T, Speck O, Grob C (2005) Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychol 162:361–369
Chang L, Ernst T, St Hillaire C, Conant K (2004) Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther 9:431–440
Chang L, Ernst T, Witt M, Ames N, Jocivich J, Speck O, Gaiefsky M, Walot I, Miller E (2002) Relationships among cerebral metabolites, cognitive function and viral loads in antiretroviral-naïve HIV patients. NeuroImage 17:1638–1648
Ciborowski P, Gendelman HE (2006) Human immunodeficiency virus-mononuclear phagocyte interactions: emerging avenues of biomarker discovery, modes of viral persistence and disease pathogenesis. Curr HIV Res 4(3):279–291
Cloak CC, Chang L, Ernst T (2004) Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. J Neuroimmunol 157:147–152
Conant K, Garzino-Demo A, Nath A, McArthur J, Halliday W, Power C, Gallo R, Major E (1998) Induction of monocyte chemoattractant protein-1 in HIV-1 tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA 95:3117–3121
D’Aversa T, Yu KO, Berman JW (2004) Expression of chemokines by human fetal microglia after treatment with the human immunodeficiency virus type 1 protein tat. J Neurovirology 10(2):86–97
D’Aversa TG, Eugenin EA, Berman JW (2005) NeuroAIDS: contributions of the human immunodeficiency virus-1 proteins tat and gp120 as well as CD40 to microglial activation. J Neurosci Res 81(3):436–446
Dafny N (1998) Is interferon-alpha a neuromodulator? Brain Res Brain Res Rev 26:1–15
Dafny N, Yang PB (2005) Interferon and the central nervous system. Eur J Pharmacol 523:1–15
Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T (2006) Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci USA 103:7835–7840
Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, Gorantla S, Poluektova L, Nelson JA, Chaubal M, Werling J, Kipp J, Rabinow B, Gendelman HE (2006) Development of a macrophage-based nanoparticle system for anti-retroviral drug delivery. Blood 108(8):2827–2835
Eisenstein TK, Bussiere JL, Rogers TJ, Adler MW (1993) Immunosuppressive effects of morphine on immune responses in mice. Adv Exp Med Biol 335:41–52
Eledrisi MS, Verghese AC (2001) Adrenal insufficiency in HIV infection: a review and recommendations. Am J Med Sci 321:137–144
El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF (2005) Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia 50:91–106
El-Hage N, Wu G, Ambati J, Bruce-Keller AJ, Knapp PE, Hauser KF (2006a) CCR2 mediates increases in glial activation caused by exposure to HIV-1 Tat and opiates. J Neuroimmunol 178(1–2):9–16
El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF (2006b) HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia 53:132–146
Ernst T, Chang L, Arnold S (2003) Increased glial markers predict increased working memory network activation in HIV patients. NeuroImage 19:1686–1693
Eugenin EA, Dyer G, Calderon TM, Berman JW (2005) HIV-1 tat protein induces a migratory phenotype in human fetal microglia by a CCL2 (MCP-1) dependent mechanism: a possible role in NeuroAIDS. Glia 49:501–510
Eugenin EA, Gamss R, Buckner C, Buono D, Klein RS, Schoenbaum EE, Calderon TM, Berman JW (2006a) Shedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDS. J Leukoc Biol 79:444–452
Eugenin EA, Osieki K, Lopez L, Goldstein H, Calderon TM, Berman JW (2006b) CCL2/Monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood–brain barrier: a potential mechanisms of HIV–CNS invasion and NeuroAIDS. J Neurosci 26(4):1098–1106
Everall I, Barnes H, Spargo E, Lantos P (1995) Assessment of neuronal density in the putamen in human immunodeficiency virus (HIV) infection. Application of stereology and spatial analysis of quadrats. J Neurovirology 1:126–129
Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E (1999) Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol 9:209–217
Fiala M, Eshleman AJ, Cashman J, Lin J, Lossinsky AS, Suarez V, Yang W, Zhang J, Popik W, Singer E, et al. (2005) Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J Neurovirology 11:281–291
Fleckenstein AE, Hanson GR (2003) Impact of psychostimulants on vesicular monoamine transporter function. Eur J Pharmacol 479:283–289
Fox HS, Weed MR, Huitron-Resendiz S, Baig J, Horn TFW, Dailey PJ, Bischofberger N, Henriksen SJ (2000) Normalization of CNS evoked potential but not movement abnormalities by antiviral treatment in SIV-infected monkeys. J Clin Invest 106:37–45
Freda PU, Bilezikian JP (1999) The hypothalamus–pituitary–adrenal axis in HIV disease. AIDS Read 9:43–50
Fujimura RK, Goodkin K, Petito CK, Douyon R, Feaster DJ, Concha M, Shapshak P (1997) HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia. J Acquir Immune Defic Syndr Human Retrovirol 16:146–152
Gallo V, Chittajallu R (2001) Neuroscience: unwrapping glial cells from the synapse: what lies inside? Science 292:872–873
Gao H, Hong JS, Zhang W, Liu B (2002) Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790
Gavrilin MA, Mathes LE, Podell M (2002) Methamphetamine enhances cell-associated feline immunodeficiency virus replication in astrocytes. J Neurovirology 8:240–249
Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, Kulkarni H, Bamshad MJ, Telles V, Anderson SA, Walter EA, Stephan KT, Deucher M, Mangano A, Bologna R, Ahuja SS, Dolan MJ, Ahuja SK (2002) HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci USA 99:13795–13800
Hanson GR, Rau KS, Fleckenstein AE (2004a) The Methamphetamine Experience: a NIDA Partnership. Neuropharmacology 47:92–100
Hanson GR, Sandoval V, Riddle E, Fleckenstein AE (2004b) Psychostimulant and vesicle trafficking: a novel mechanism and therapeutic implications. Ann NY Acad Sci 1025:146–150
Hauser KF, El-Hage N, Buch S, Berger JR, Tyor WR, Nath A, Bruce-Keller AJ, Knapp PE (2005) Molecular targets of opiate drug abuse in NeuroAIDS. Neurotox Res 8:63–80
Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE (1998) Beta-Chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol 44:831–835
Kim WG, Wilson BC, Jeohn GH, Hong JS (2000) Differential susceptivity of neurons in different brain regions in lipopolysaccharide (LPS)-induced neurotoxicity. J Neurosci 20:6309–6316
King JE, Eugenin EA, Buckner CM, Berman JW (2006) HIV tat and neurotoxicity. Microbes Infect 8(5):1347–1357
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, et al (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 55:306–319
Kopnisky KL, Stoff DM, Rausch DM (2004) Workshop report: the effects of psychological variables on the progression of HIV-1 disease. Brain Behav Immun 18:246–261
Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P (2002) Involvement of dopamine in the progression of AIDS dementia complex. J Neural Transm 109:399–410
Kumar M, Kumar AM, Waldrop D, Antoni MH, Eisdorfer C (2003) HIV-1 infection and its impact on the HPA axis, cytokines, and cognition. Stress 6:167–172
Langer J, Garotta G, Pestka S (1996) Interferon receptors. Biotherapy 8:163–174
Laudenbach V, Calo G, Guerrini R, Lamboley G, Bernoist J-F, Evrard P, Gressens P (2001) Nociceptin/orphanin FQ exacerbates excitotoxic white-matter lesions in the murine neonatal brain. J Clin Invest 1007:457–466
Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO (2004) Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol 78:7319–7328
Lawrence DM, Seth P, Durham L, Diaz F, Boursiquot R, Ransohoff RM, Major EO (2006) Astrocyte differentiation selectively upregulates CCL2/monocyte chemoattractant protein-1 in cultured human brain-derived progenitor cells. Glia 53:81–91
Lentz MR, Kim JP, Westmoreland SV, Greco JB, Fuller RA, Ratai EM, He J, Sehgal PK, Halpern F, Lackner AA, Masliah E, Gonzalez RG (2005) Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology 235:461–468
Liu Y, Qin L, Wilson B, Hong JS, Liu B (2002) Inhibition of naloxone stereoisomers of β-amyloid peptide-induced superoxide production in microglia and degeneration of cortical and mesencephalic neurons. J Pharmacol Exp Ther 302:1212–1219
Madden LJ, Flynn CT, Zandonatti MA, May M, Parsons LH, Katner SH, Henriksen SJ, Fox HS (2005) Modeling human methamphetamine exposure in non-human primates: chronic dosing in the rhesus macaque leads to behavioral and physiological abnormalities. Neuropsychopharmacology 30:350–359
Mahajan SD, Schwartz SA, Shanahan TC, Chawda RP, Nair MP (2002) Morphine regulates gene expression of alpha- and beta-chemokines and their receptors on astroglial cells via the opioid mu receptor. J Immunol 169:3589–3599
Maragos WF, Young KL, Altman CA, Turchan JT, Pauly, JR, Guseva M, Nath A, Cass WA (2002) HIV-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem 83:955–963
Marcondes MCG, Burudi EME, Huitron-Resendiz S, Sanchez-Alvarez M, Watry D, Zandonatti M, Henriksen SJ, Fox HS (2001) Highly activated CD8+ T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol 167:5429–5438
Marti M, Guerrini R, Beani L, Bianchi C, Morari M (2002) Nocicpetin/orphanin FQ receptors modulate glutamate extracellular levels in the substania nigra pars reticulata. A microdialysis study in the awake freely moving rat. Neuroscience 112:153–160
Marti M, Mela F, Fantin M, Zucchini S, Brown JM, Witta J, Di Benedetto M, Buzas B, Reinscheid RK, Salvadori S, Guerrini R, Romualdi P, Candeletti S, Simonato M, Cox BM, Morari M (2005) Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson’s disease. J Neurosci 25:9591–9601
Masliah E, Ge N, Achim CL, Hansen LA, Wiley CA (1992) Selective neuronal vulnerability in HIV encephalitis. J Neuropathol Exp Neurol 51:585–593
Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I (1997) Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. Ann Neurol 42:963–972
McArthur JC, Brew BJ, Nath A (2005) Neurological complications of HIV infection. Lancet Neurol 4:543–555
Messam CA, Hou J, Gronostajski RM, Major EO (2003) Lineage pathway of human brain progenitor cells identified by JC virus susceptibility. Ann Neurol 53:636–646
Miller DB, O’Callaghan JP (2002) Neuroendocrine aspects of the response to stress. Metabolism 51(Suppl 1):5–10
Miller DB, O’Callaghan JP (2005) Aging, stress and the hippocampus. Ageing Res Rev 4:123–140
Nair MP, Mahajan S, Hewitt R, Whitney ZR, Schwartz SA (2004) Association of drug abuse with inhibition of HIV-1 immune responses: studies with long-term of HIV-1 non-progressors. J Neuroimmunol 147:21–25
Nair MP, Schwartz SA, Polasani R, Hou J, Sweet A, Chadha KC (1997) Immunoregulatory effects of morphine on human lymphocytes. Clin Diagn Lab Immunol 4:127–132
Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT (2002) Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr 31(Suppl 2):S62–S69
Nath A, Jones M, Maragos W, Booze RM, Mactutus C, Bell J, Mattson M (2000) Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol 14:222–227
Navia BA, Cho ES, Petito CK, Price RW (1986) The AIDS dementia complex: II. Neuropathology. Ann Neurol 19:525–535
O’Callaghan JP, Sriram K (2005) GFAP and other glial proteins as biomarkers of neurotoxicity. Exp Opin Drug Saf 4:433–442
Pandya R, Krentz HB, Gill MJ, Power C (2005) HIV-related neurological syndromes reduce health-related quality of life. Can J Neurol Sci 32:201–204
Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T (2006b) Rho-mediated regulation of tight junctions during monocyte migration across the blood–brain barrier in HIV-1 encephalitis (HIVE). Blood 107:4770–4780
Persidsky Y, Ramirez S, Haorah J, Kanmogne G (2006a) Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 1:223–236
Pestka S, Langer JA, Zoon KC, Samuel CE (1987) Interferons and their actions. Ann Rev Biochem 56:727–777
Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC (1999) Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implication of an atypical mu-opioid receptor. Neuropharmacology 38:273–278
Peterson PK, Sharp B, Gekker G, Brummitt C, Keane WF (1987) Opioid-mediated suppression of interferon-gamma production by cultured peripheral blood mononuclear cells. J Clin Invest 80:824–831
Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH Jr (1990) Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS 4:869–873
Potula R, Poluektova L, Knipe B, Chrastil J, Heilman D, Dou H, Takikawa O, Munn DH, Gendelman HE, Persidsky Y (2005) Inhibition of Indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in animal model of HIV-1 encephalitis. Blood 106:2383–2390
Qin L, Block M, Liu Y, Bienstock R, Pei Z, Zhang W, Wu X, Wilson B, Burka T, Hong JS (2005) Microglial NADPH oxidase is a novel target for femtomolar neuroprotection against oxidative stress. FASEB J 19:550–557
Rau K, Birdsall E, Volz T, Riordan J, Baucum A, Adair B, Bitter R, Gibb JW, Hanson GR, Fleckenstein AE (2006) Methamphetamine administration reduces hippocampal VMAT-2 uptake. J Pharmacol Exp Ther 318:676–682
Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R (1991) Nigral degeneration in acquired immune deficiency syndrome (AIDS). Acta Neuropathol 82:39–44
Riddle E, Fleckenstein AE, Hanson GR (2006) Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. J Am Assoc Pharm Sci 8:E413–E418
Riddle EL, Fleckenstein AE, Hanson GR (2005) Role of monoamine transporters in mediating psychostimulant effects. J Am Assoc Pharm Sci 7:E847–E851
Riddle EL, Topham M, Haycock J, Hanson G, Fleckenstein A (2002) Differential trafficking of the vesicular monoamine transporter-2 by methamphetamine and cocaine. Eur J Pharmacol 449:71–74
Roberts ES, Burudi EME, Flynn C, Madden LJ, Roinick KL, Watry DD, Zandonatti MA, Taffe MA, Fox HS (2004a) Acute SIV infection of the brain leads to upregulation of IL6 and interferon-regulated genes: expression patterns throughout disease progression and impact on NeuroAIDS. J Neuroimmunol 157:81–92
Roberts ES, Huitron-Resendiz S, Taffe MA, Marcondes MC, Flynn CT, Lanigan CM, Hammond JA, Head SR, Henriksen SJ, Fox HS (2006) Host response and dysfunction in the CNS during chronic SIV infection. J Neurosci 26:4577–4585
Roberts ES, Masliah E, Fox HS (2004b) CD163 identifies a unique population of ramified microglia in HIV encephalitis. J Neuropathol Exp Neurol 63:1255–1264
Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA, Fox HS (2003) Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol 162:2041–2057
Rogers TJ, Peterson PK (2003) Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol 24:116–121
Sloan EK, Tarara RP, Capitanio JP, Cole SW (2006) Enhanced replication of simian immunodeficiency virus adjacent to catecholaminergic varicosities in primate lymph nodes. J Virol 80:4326–4335
Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417:39–44
Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP (2006a) Microglial activation and MPTP-induced neurotoxicity: region-specific role for TNF-α. FASEB J 20:670–682
Sriram K, Miller DB, O’Callaghan JP (2006b) Minocyline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of TNF-α. J Neurochem 96:706–719
Steele AD, Henderson EE, Rogers TJ (2003) Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology 309:99–107
Steele AD, Szabo I, Bednar F, Rogers TJ (2002) Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine Growth Factor Rev 13:209–222
Stiene-Martin A, Knapp PE, Martin KM, Gurwell JA, Ryan S, Thornton SR, Smith FL, Hauser KF (2001) Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia 36:78–88
Stiene-Martin A, Zhou R, Hauser KF (1998) Regional, developmental, and cell cycle-dependent differences in mu, delta, and kappa opioid receptor expression among cultured mouse astrocytes. Glia 22:249–259
Stoll-Keller F, Schmitt C, Thumann C, Schmitt MP, Caussin C, Kirn A (1997) Effects of morphine on purified human blood monocytes. Modifications of properties involved in antiviral defences. Int J Immunopharmacol 19:95–100
Streit WJ, Walter SA, Pennell NA (1999) Reactive microgliosis. Prog Neurobiol 57:563–581
Svendsen CN (2002) The amazing astrocyte. Nature 417:29–32
Szabo I, Wetzel MA, Zhang N, Steele AD, Kaminsky DE, Chen C, Liu-Chen LY, Bednar F, Henderson EE, Howard OM, Oppenheim JJ, Rogers TJ (2003) Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. J Leukoc Biol 74:1074–1082
Theodore S, Cass WA, Maragos WF (2006a) Involvement of cytokines in human immunodeficiency virus-1 protein Tat and methamphetamine interactions in the striatum. Exp Neurol 199:490–498
Theodore S, Cass WA, Maragos WF (2006b) Methamphetamine and human immunodeficiency virus protein Tat synergize to destroy dopaminergic terminals in the rat striatum. Neuroscience 137:925–935
Theodore S, Nath A, Young K, Cass WA, Maragos WF (2006c) Inhibition of tumor necrosis factor-alpha signaling prevents human immunodeficiency virus-1 protein tat and methamphetamine interaction. Neurobiol Dis, July 5 [Epub ahead of print]
Theodore S, Stolberg S, Cass WA, Maragos WF (2006d) Human immunodeficiency virus-1 protein tat and methamphetamine interactions. Ann NY Acad Sci 1074:178–190
Turchan-Cholewo J, Liu Y, Gartner S, Reid R, Jie C, Peng X, Chen K, Chauhan A, Haughey N, Cutler R, Mattson MP, Pardo C, Conant K, Sacktor N, McArthur J, Hauser KF, Gairola C, Nath A (2006) Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and l-deprenyl. Neurobiol Dis 23:109–119
van Marle G, Power C (2005) Human immunodeficiency virus type 1 genetic diversity in the nervous system: evolutionary epiphenomenon or disease determinant? J Neurovirology 11:107–128
Volkow N, Chang L, Wang G, Fowler J, Ding Y, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, et al (2001a) Low level of brain dopamine d(2) receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychol 158:2015–2021
Volkow N, Chang L, Wang G, Fowler J, Leonido-Yee M, Franceschi D, Sedler M, Gatley S, Hitzemann R, Ding Y, et al (2001b) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychol 158:377–382
Volz TJ, Hanson GR, Fleckenstein AE (2006) Measurement of kinetically resolved vesicular dopamine uptake and efflux using rotating disk electrode voltammetry. J Neurosci Methods 155:109–115
Wang G, Chang L, Volkow N, Telang F, Logan J, Ernst T, Fowler J (2004) Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain 127:2452–2458
Webster JI, Sternberg EM (2004) Role of the hypothalamic–pituitary–adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J Endocrinol 181:207–221
Wetzel MA, Steele AD, Eisenstein TK, Adler MW, Henderson EE, Rogers TJ (2000) Mu-opioid induction of monocyte chemoattractant protein-1, RANTES, and IFN-gamma-inducible protein-10 expression in human peripheral blood mononuclear cells. J Immunol 165:6519–6524
Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG (2005) Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV NeuroAIDS. J Clin Invest 115:2534–2545
Witta J, Buzas B, Cox BM (2003) Traumatic brain injury induces nociceptin/orphanin FQ expression in neurons of the rat cerebral cortex. J Neurotrauma 20(6):523–532
Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S (2002) Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 22:1763–1771
Zhang W, Qin L, Wang T, Wei S, Gao H, Liu J, Wilson B, Liu B, Zhang W, Kim H, Hong JS (2005) 3-Hydroxy-morphinan is neurotrophic to dopaminergic neurons and is also neuroprotective against LPS-induced neurotoxicity. FASEB J 19:395–397
Acknowledgment
The authors offer a particular debt of gratitude for the outstanding guidance, support and organizational skills of Diane Lawrence and Lynda Erinoff who made the meeting and transcripts possible. The authors wish to acknowledge Roy Walker, Project Manager from CSR, Incorporated, for his outstanding efforts in coordinating and moderating the workshop, as well as Nellie Theresa Ingraham, Connie Curro, and Sandra Wiese for transcription of the presentations. A special thank you is made to Dr. Howard E. Gendelman and Ms. Robin Taylor for critical readings of the manuscript constant encouragement of the work and seeing it to its successful conclusion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berman, J.W., Carson, M.J., Chang, L. et al. NeuroAIDS, Drug Abuse, and Inflammation: Building Collaborative Research Activities. Jrnl Neuroimmune Pharm 1, 351–399 (2006). https://doi.org/10.1007/s11481-006-9048-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-006-9048-9