Abstract
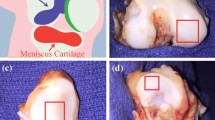
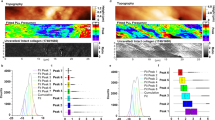
Joint disease affects approximately 52.5 million patients in the United States alone, costing 80.8 billion USD in direct healthcare costs. The development of treatment programs for joint disease and trauma requires accurate assessment of articular cartilage degradation. The articular cartilage is the interfacial tissue between articulating surfaces, such as bones, and acts as low-friction interfaces. Damage to the lamina splendens, which is the articular cartilage’s topmost layer, is an early indicator of joint degradation caused by injury or disease. By gaining comprehensive knowledge on the lamina splendens, particularly its structure and interfacial properties, researchers could enhance the accuracy of human and animal biomechanical models, as well as develop appropriate biomimetic materials for replacing damaged articular cartilage, thereby leading to rational treatment programs for joint disease and injury. Previous studies that utilize light, electron, and force microscopy techniques have found that the lamina splendens is composed of collagen fibers oriented parallel to the cartilage surface and encased in a proteoglycan matrix. Such orientation maximizes wear resistance and proteoglycan retention while promoting the passage of nutrients and synovial fluid. Although the structure of the lamina splendens has been explored in the literature, the low-friction interface of this tissue remains only partially characterized. Various functional models are currently available for the interface, such as pure boundary lubrication, thin films exuded under pressure, and sheets of trapped proteins. Recent studies suggest that each of these lubrication models has certain advantages over one another. Further research is needed to fully model the interface of this tissue. In this review, we summarize the methods for characterizing the lamina splendens and the results of each method. This paper aims to serve as a resource for existing studies to date and a roadmap of the investigations needed to gain further insight into the lamina splendens and the progression of joint disease.
Similar content being viewed by others
References
Centers for Disease Control and Prevention. Osteoarthritis, 2014, http://www.cdc.gov/arthritis/basics/osteoarthritis.htm
Centers for Disease Control and Prevention. Arthritis: Cost Statistics. 2015, http://www.cdc.gov/arthritis/data_statistics/cost.htm
Centers for Disease Control and Prevention. Arthritis: National Statistics. 2016, http://www.cdc.gov/arthritis/data_statistics/national-statistics.html
Desrochers J, Amrein M A, Matyas J R. Structural and functional changes of the articular surface in a post-traumatic model of early osteoarthritis measured by atomic force microscopy. Journal of Biomechanics, 2010, 43(16): 3091–3098
Weiss C, Mirow S. An ultrastructural study of osteoarthritic changes in the articular cartilage of human knees. The Journal of Bone and Joint Surgery. American Volume, 1972, 54(5): 954–972
Hollander A P, Dickinson S C, Kafienah W. Stem cells and cartilage development: Complexities of a simple tissue. Stem Cells, 2010, 28(11): 1992–1996
Jay G D, Torres J R, Rhee D K, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis & Rheumatism, 2007, 56(11): 3662–3669
Wu J P, Kirk T B, Zheng M H. Assessment of three-dimensional architecture of collagen fibers in the superficial zone of bovine articular cartilage. Journal of Musculoskeletal Research, 2004, 08(04): 167–179
Thambyah A, Broom N. On how degeneration influences loadbearing in the cartilage-bone system: A microstructural and micromechanical study. Osteoarthritis and Cartilage, 2007, 15(12): 1410–1423
MacConaill M A. The movements of bones and joints. Journal of Bone and Joint Surgery, 1951, 33-B: 251–257
Aspden R M, Hukins D W L. The lamina splendens of articular cartilage is an artefact of phase contrast microscopy. Proceedings of the Royal Society of London. Series B, Biological Sciences, 1979, 206(1162): 109–113
Clark J M. The organisation of collagen fibrils in the superficial zones of articular cartilage. Journal of Anatomy, 1990, 171: 117–130
Weiss C, Rosenberg L, Helfet A J. An ultrastructural study of normal young adult human articular cartilage. Journal of Bone and Joint Surgery. American Volume, 1968, 50(4): 663–674
Cohen N P, Foster R J, Mow V C. Composition and dynamics of articular cartilage: Structure, function, and maintaining healthy state. Journal of Orthopaedic & Sports Physical Therapy, 1998, 28(4): 203–215
Wu J P, Kirk T B, Zheng M H. Study of the collagen structure in the superficial zone and physiological state of articular cartilage using a 3D confocal imaging technique. Journal of Orthopaedic Surgery and Research, 2008, 3(29): 1–11
Teshima R, Otsuka T, Takasu N, et al. Structure of the most superficial layer of articular cartilage. The Journal of Bone and Joint Surgery. British Volume, 1995, 77(3): 460–464
Jeffery A K, Blunn G W, Archer C W, et al. Three-dimensional collagen architecture in bovine articular cartilage. Journal of Bone and Joint Surgery. British Volume, 1991, 73(5): 795–801
Clarke I C. Articular cartilage: A review and scanning electron microscope study. Journal of Bone and Joint Surgery. British Volume, 1971, 53(4): 732–750
Teshima R, Ono M, Yamashita Y, et al. Immunohistochemical collagen analysis of the most superficial layer in adult articular cartilage. Journal of Orthopaedic Science, 2004, 9(3): 270–273
Fujioka R, Aoyama T, Takakuwa T. The layered structure of the articular surface. Osteoarthritis and Cartilage, 2013, 21(8): 1092–1098
Coles J M, Zhang L, Blum J J, et al. Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis and Rheumatism, 2010, 62(6): 1666–1674
Jurvelin J S, Müller D J, Wong M, et al. Surface and subsurface morphology of bovine humeral articular cartilage as assessed by atomic force and transmission electron microscopy. Journal of Structural Biology, 1996, 117(1): 45–54
Mansour J M. Biomechanics of Cartilage. In: Hughes C, ed. Kinesiology: The Mechanics and Pathomechanics of Human Movement. 2nd ed. Baltimore: Lippincott Williams & Wilkins, 2009, 66–79
Dunham J, Shackleton D R, Billingham ME J, et al. A reappraisal of the structure of normal canine articular cartilage. Journal of Anatomy, 1988, 157: 89–99
Davies D V, Barnett C H, Cochrane W, et al. Electron microscopy of articular cartilage in the young adult rabbit. Annals of the Rheumatic Diseases, 1962, 21(1): 11–22
Jay G D, Torres J R, Warman M L, et al. The role of lubricin in the mechanical behavior of synovial fluid. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104 (15): 6194–6199
Balazs E A, Bloom G A, Swann D A. Fine structure and glycosaminoglycan content of the surface layer of articular cartilage. Federation Proceedings, 1966, 25(6): 1813–1816
Walker P S, Sikorski J, Dowson D, et al. Behaviour of synovial fluid on surfaces of articular cartilage. A scanning electron microscope study. Annals of the Rheumatic Diseases, 1969, 28(1): 1–14
Aspden R M. Cartilage Structure.https://www.abdn.ac.uk/ims/research/musculoskeletal/cartilage-structure-1197.php
Silva C, Horkayne-Szakaly I, Lin D C, et al. Osmotic swelling behavior of bovine cartilage. Proceedings of the 238th ACS National Meeting. American Chemical Society. Polymer Preprints, 2009, 50(2): 553–554
von der Mark K, Park J, Bauer S, et al. Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell and Tissue Research, 2010, 339(1): 131–153
Krishnan R, Park S, Eckstein F, et al. Inhomogeneous cartilage properties enhance superficial interstitial fluid support and frictional properties, but do not provide a homogeneous state of stress. Journal of Biomechanical Engineering, 2003, 125(5): 569–577
Basalo I M, Raj D, Krishnan R, et al. Effects of enzymatic degradation on the frictional response of articular cartilage in stress relaxation. Journal of Biomechanics, 2005, 38(6): 1343–1349
O’Hara B P, Urban J P G, Maroudas A. Influence of cyclic loading on the nutrition of articular cartilage. Annals of the Rheumatic Diseases, 1990, 49(7): 536–539
Das S, Banquy X, Zappone B, et al. Synergistic interactions between grafted hyaluronic acid and lubricin provide enhanced wear protection and lubrication. Biomacromolecules, 2013, 14(5): 1669–1677
Elsaid K A, Chichester C O, Jay G D. Lubricin purified from bovine synovial fluid and from articular cartilage exhibit similar binding affinities to cartilage matrix proteins. In: Proceedings of 53rd Annual Meeting of the Orthopaedic Research Society. Poster Abstract, 2007
Chang D P, Abu-Lail N I, Coles J M, et al. Friction force microscopy of lubricin and hyaluronic acid between hydrophobic and hydrophilic surfaces. Soft Matter, 2009, 5(18): 3438–3445
Chang D P, Abu-Lail N I, Guilak F, et al. Conformational mechanics, adsorption, and normal force interactions of lubricin and hyaluronic acid on model surfaces. Langmuir, 2008, 24(4): 1183–1193
Bonnevie E D, Galesso D, Secchieri C, et al. Elastoviscous transitions of articular cartilage reveal a mechanism of synergy between lubricin and hyaluronic acid. PLoS ONE, 2015, 10(11): e043415
Sypeck D. Damage evolution in titanium matrix composites. Dissertation for the Doctoral Degree. Charlottesville: University of Virginia, 1996
Ministry of Defence, England. Royal armament research and development establishment. In: Watson-Adams B R, Dibb J J, Wronski A S, eds. Mechanical Properties of Fiber-Reinforced Composites Tested Under Superposed Hydrostatic Pressures. VA: National Technical Information Services, 1975
Caligaris M, Ateshian G A. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthritis and Cartilage, 2008, 16(10): 1220–1227
Chan S M T, Neu C P, Duraine G, et al. Tribological altruism: A sacrificial layer mechanism of synovial joint lubrication in articular cartilage. Journal of Biomechanics, 2012, 45(14): 2426–2431
Jay G D. Lubricin and Surfacing of Articular Joints. Current Opinion in Orthopaedics, 2004, 15(5): 355–359
Guilak F, Ratcliffe A, Mow V C. Chondrocyte deformation and local tissue strain in articular cartilage: A confocal microscopy study. Journal of Orthopaedic Research, 1995, 13(3): 410–421
Sung K B, Richards-Kortum R, Follen M, et al. Fiber optic confocal reflectance microscopy: A new real-time technique to view nuclear morphology in cervical squamous epithelium in vivo. Optics Express, 2003, 11(24): 3171–3181
Yeh A T, Hammer-Wilson M J, Van Sickle D C, et al. Nonlinear optical microscopy of articular cartilage. Osteoarthritis and Cartilage, 2005, 13(4): 345–352
Hanson K M, Bardeen C J. Application of nonlinear optical microscopy for imaging skin. Photochemistry and Photobiology, 2009, 85(1): 33–44
Kumar P, Oka M, Toguchida J, et al. Role of uppermost superficial surface layer of articular cartilage in the lubrication mechanism of joints. Journal of Anatomy, 2001, 199(3): 241–250
Kobayashi S, Yonekubo S, Kurogouchi Y. Cryoscanning electron microscopy of loaded articular cartilage with special reference to the surface amorphous layer. Journal of Anatomy, 1996, 188(Pt2): 311–322
Crockett R, Roos S, Rossbach P, et al. Imaging of the surface of human and bovine articular cartilage with ESEM and AFM. Tribology Letters, 2005, 19(4): 311–317
Chan S M T, Neu C P, Duraine G, et al. Atomic force microscope investigation of the boundary-lubricant layer in articular cartilage. Osteoarthritis and Cartilage, 2010, 18(7): 956–963
Han L, Frank E H, Greene J J, et al. Time-dependent nanomechanics of cartilage. Biophysical Journal, 2011, 100(7): 1846–1854
Desrochers J, Amrein M W, Matyas J R. Viscoelasticity of the articular cartilage surface in early osteoarthritis. Osteoarthritis and Cartilage, 2012, 20(5): 413–421
Park S, Costa K D, Ateshian G A. Microscale frictional response of bovine articular cartilage from atomic force microscopy. Journal of Biomechanics, 2004, 37(11): 1679–1687
Moa-Anderson B J, Costa K D, Hung C T, et al. Bovine articular cartilage surface topography and roughness in fresh versus frozen tissue samples using atomic force microscopy. In: Proceedings of Summer Bioengineering Conference. New Orleans, 2003
Chan S M T, Neu C P, Komvopoulos K, et al. Dependence of nanoscale friction and adhesion properties of articular cartilage on contact load. Journal of Biomechanics, 2011, 44(7): 1340–1345
Bae WC, Temple MM, Amiel D, et al. Indentation testing of human cartilage: Sensitivity to articular surface degeneration. Arthritis and Rheumatism, 2003, 48(12): 3382–3394
Caligaris M, Canal C E, Ahmad C S, et al. Investigation of the frictional response of osteoarthritic human tibiofemoral joints and the potential beneficial tribological effect of healthy synovial fluid. Osteoarthritis and Cartilage, 2009, 17(10): 1327–1332
Schmidt T A, Gastelum N S, Nguyen Q T, et al. Boundary lubrication of articular cartilage: Role of synovial fluid constituents. Arthritis and Rheumatism, 2007, 56(3): 882–891
Krishnan R, Caligaris M, Mauck R L, et al. Removal of the superficial zone of bovine articular cartilage does not increase its frictional coefficient. Osteoarthritis and Cartilage, 2004, 12(12): 947–955
Chan S M T, Neu C P, Komvopoulos K, et al. The role of lubricant entrapment at biological interfaces: Reduction of friction and adhesion in articular cartilage. Journal of Biomechanics, 2011, 44 (11): 2015–2020
Greene G W, Banquy X, Lee D W, et al. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(13): 5255–5259
Schmidt T A, Sah R L. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis and Cartilage, 2007, 15(1): 35–47
Basalo I M, Chen F H, Hung C T, et al. Frictional response of bovine articular cartilage under creep loading following proteoglycan digestion with chondroitinase ABC. Journal of Biomechanical Engineering, 2006, 128(1): 131–134
John Innes Center. What is light microscopy? https://www.jic.ac.uk/microscopy/intro_LM.html
Shackleton D R, Nahir A M, Billingham M E J, et al. The lamina splendens of articular cartilage: Fact or artifact. Clinical Science, 1984, 66(6): 22
University of Utah. Electron Microscopy Tutorial. http://advancedmicroscopy.utah.edu/education/electron-micro/index.html
University of Iowa. Transmission Electron Microscopy. https://cmrf.research.uiowa.edu/transmission-electron-microscopy
University of Iowa. Scanning Electron Microscopy. https://cmrf.research.uiowa.edu/scanning-electron-microscopy
Sargent J A. Low temperature scanning electron microscopy: Advantages and applications. Scanning Microscopy, 1988, 2(2): 835–849
Donald A M. The use of environmental scanning electron microscopy for imaging wet and insulating materials. Nature Materials, 2003, 2(8): 511–516
Meyer E. Atomic force microscopy. Progress in Surface Science, 1992, 41(1): 3–49
Chan S M T, Neu C P, Komvopoulos K, et al. Dependence of nanoscale friction and adhesion properties of articular cartilage on contact load. Journal of Biomechanics, 2011, 44(7): 1340–1345
Mitchell N, Laurin C, Shepard N. The effect of osmium tetroxide and nitrogen mustard on normal articular cartilage. Journal of Bone and Joint Surgery. British Volume, 1973, 55(4): 814–821
Hollander A P, Pidoux I, Reiner A, et al. Damage to Type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. Journal of Clinical Investigation, 1995, 96(6): 2859–2869
Sun Y, Chen M Y, Zhao C, et al. The effect of hyaluronidase, phospholipase, lipid solvent and trypsin on the lubrication of canine flexor digitorum profundus tendon. Journal of Orthopaedic Research, 2008, 26(9): 1225–1229
Muir H. Molecular approach to the understanding of osteoarthrosis. Annals of the Rheumatic Diseases, 1977, 36(3): 199–208
Banquy X, Lee D W, Das S, et al. Shear-induced aggregation of mammalian synovial fluid components under boundary lubrication conditions. Advanced Functional Materials, 2014, 24(21): 3152–3161
Israelchvili J, Min Y, Akbulut M, et al. Recent advances in the surface forces apparatus (SFA) technique. Reports on Progress in Physics, 2010, 73(3): 036601
Andresen Eguiluz R C, Cook S G, Brown C N, et al. Fibronectin mediates enhanced wear protection of lubricin during shear. Biomacromolecules, 2015, 16(9): 2884–2894
Hou J S, Holmes M H, Lai W M, et al. Boundary conditions at the cartilage-synovial fluid interface for joint lubrication and theoretical verifications. Journal of Biomechanical Engineering, 1989, 111(1): 78–87
Teeple E, Elsaid K A, Jay G D, et al. Effects of supplemental intraarticular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament-deficient rat knee. American Journal of Sports Medicine, 2011, 39(1): 164–172
Acknowledgements
We thank Khanh Van Nguyen for creating all unreferenced images in the paper and Jill Jouret and Paul J. D. Whiteside for their assistance in editing the paper prior to submission. The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rexwinkle, J.T., Hunt, H.K. & Pfeiffer, F.M. Characterization of the surface and interfacial properties of the lamina splendens. Front. Mech. Eng. 12, 234–252 (2017). https://doi.org/10.1007/s11465-017-0409-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11465-017-0409-2