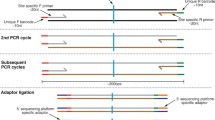
Abstract
The CRISPR/Cas system has been extensively applied to make precise genetic modifications in various organisms. Despite its importance and widespread use, large-scale mutation screening remains time-consuming, labour-intensive and costly. Here, we developed Hi-TOM (available at https://doi.org/www.hi-tom.net/hi-tom/), an online tool to track the mutations with precise percentage for multiple samples and multiple target sites. We also described a corresponding next-generation sequencing (NGS) library construction strategy by fixing the bridge sequences and barcoding primers. Analysis of the samples from rice, hexaploid wheat and human cells reveals that the Hi-TOM tool has high reliability and sensitivity in tracking various mutations, especially complex chimeric mutations frequently induced by genome editing. Hi-TOM does not require special design of barcode primers, cumbersome parameter configuration or additional data analysis. Thus, the streamlined NGS library construction and comprehensive result output make Hi-TOM particularly suitable for high-throughput identification of all types of mutations induced by CRISPR/Cas systems.
Similar content being viewed by others
References
Bell, C.C., Magor, G.W., Gillinder, K.R., and Perkins, A.C. (2014). A high-throughput screening strategy for detecting CRISPR-Cas9 induced mutations using next-generation sequencing. BMC Genomics 15, 1002.
Boel, A., Steyaert, W., De Rocker, N., Menten, B., Callewaert, B., De Paepe, A., Coucke, P., and Willaert, A. (2016). BATCH-GE: Batch analysis of Next-Generation Sequencing data for genome editing assessment. Sci Rep 6, 30330.
Brinkman, E.K., Chen, T., Amendola, M., and van Steensel, B. (2014). Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res 42, e168.
Chen, Y., Wang, Z., Ni, H., Xu, Y., Chen, Q., and Jiang, L. (2017). CRISPR/Cas9-mediated base-editing system efficiently generates gainof- function mutations in Arabidopsis. Sci China Life Sci 60, 520–523.
Goodwin, S., McPherson, J.D., and McCombie, W.R. (2016). Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet 17, 333–351.
Güell, M., Yang, L., and Church, G.M. (2014). Genome editing assessment using CRISPR Genome Analyzer (CRISPR-GA). Bioinformatics 30, 2968–2970.
Li, H., and Durbin, R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760.
Lindsay, H., Burger, A., Biyong, B., Felker, A., Hess, C., Zaugg, J., Chiavacci, E., Anders, C., Jinek, M., Mosimann, C., et al. (2016). CrispRVariants charts the mutation spectrum of genome engineering experiments. Nat Biotechnol 34, 701–702.
Metzker, M.L. (2010). Sequencing technologies—the next generation. Nat Rev Genet 11, 31–46.
Park, J., Lim, K., Kim, J.S., and Bae, S. (2016). Cas-analyzer: an online tool for assessing genome editing results using NGS data. Bioinformatics 33, 286–288.
Pinello, L., Canver, M.C., Hoban, M.D., Orkin, S.H., Kohn, D.B., Bauer, D. E., and Yuan, G.C. (2016). Analyzing CRISPR genome-editing experiments with CRISPResso. Nat Biotechnol 34, 695–697.
Ran, Y., Liang, Z., and Gao, C. (2017). Current and future editing reagent delivery systems for plant genome editing. Sci China Life Sci 60, 490–505.
Jiao, R., and Gao, C. (2017). Anything impossible with CRISPR/Cas9? Sci China Life Sci 60, 445–446.
Rodríguez-Leal, D., Lemmon, Z.H., Man, J., Bartlett, M.E., and Lippman, Z.B. (2017). Engineering quantitative trait variation for crop improvement by genome editing. Cell 171, 470–480.
Shen, L., Hua, Y., Fu, Y., Li, J., Liu, Q., Jiao, X., Xin, G., Wang, J., Wang, X., Yan, C., et al. (2017). Rapid generation of genetic diversity by multiplex CRISPR/Cas9 genome editing in rice. Sci China Life Sci 60, 506–515.
Wang, Y., Cheng, X., Shan, Q., Zhang, Y., Liu, J., Gao, C., and Qiu, J.L. (2014). Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol 32, 947–951.
Winter, J., Breinig, M., Heigwer, F., Brügemann, D., Leible, S., Pelz, O., Zhan, T., and Boutros, M. (2016). caRpools: an R package for exploratory data analysis and documentation of pooled CRISPR/Cas9 screens. Bioinformatics 32, 632–634.
Xie, X., Ma, X., Zhu, Q., Zeng, D., Li, G., and Liu, Y.G. (2017). CRISPRGE: a convenient software toolkit for cRISPR-based genome editing. Mol Plant 10, 1246–1249.
Xue, L.J., and Tsai, C.J. (2015). AGEseq: analysis of genome editing by sequencing. Mol Plant 8, 1428–1430.
Zhang, H., Zhang, J., Wei, P., Zhang, B., Gou, F., Feng, Z., Mao, Y., Yang, L., Zhang, H., Xu, N., et al. (2014). The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol J 12, 797–807.
Zhang, X., Wang, L., Liu, M., and Li, D. (2017). CRISPR/Cas9 system: a powerful technology for in vivo and ex vivo gene therapy. Sci China Life Sci 60, 468–475.
Acknowledgements
We are extremely grateful to Ruiqiang Li from Novogene Bioinformatics Institute for critical reading of the manuscript. We thank Zheng Ruan and his team from Novogene Co., Ltd for NGS technical service. We also thank Yangwen Qian from Hangzhou Biogle Co., Ltd for rice transformation. This work was supported by the National Key Research and Development Program of China (2017YFD0102002), the Agricultural Science and Technology Innovation Program of Chinese Academy of Agricultural Sciences, and the National Natural Science Foundation of China (31401363).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, Q., Wang, C., Jiao, X. et al. Hi-TOM: a platform for high-throughput tracking of mutations induced by CRISPR/Cas systems. Sci. China Life Sci. 62, 1–7 (2019). https://doi.org/10.1007/s11427-018-9402-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-018-9402-9