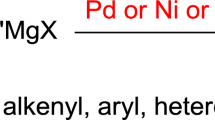Abstract
A radical-mediated method was reported for diastereoselective synthesis of non-classical heteroaryl C-glycosides via Minisci-type alkylation of N-heteroarenes with 4-glycosyl-1,4-dihydropyridine (DHP) reagents. These DHP reagents serve as convenient precursors for various glycosyl radicals under the activation of single electron transfer (SET) oxidation by persulfate and visible light irradiation with or without photocatalyst.

Similar content being viewed by others
References
Reviews on synthesis and biological application of C-aryl glycosides: Jaramillo C, Knapp S. Synthesis, 1994, 1994(01): 1–20
Bililign T, Griffith BR, Thorson JS. Nat Prod Rep, 2005, 22: 742–760
Frihed TG, Bols M, Pedersen CM. Eur J Org Chem, 2016, 2016(16): 2740–2756
Yang Y, Yu B. Chem Rev, 2017, 117: 12281–12356
Kitamura K, Ando Y, Matsumoto T, Suzuki K. Chem Rev, 2018, 118: 1495–1598
Aguillón AR, Mascarello A, Segretti ND, de Azevedo HFZ, Guimaraes CRW, Miranda LSM, de Souza ROMA. Org Process Res Dev, 2018, 22: 467–488
Goodwin NC, Mabon R, Harrison BA, Shadoan MK, Almstead ZY, Xie Y, Healy J, Buhring LM, DaCosta CM, Bardenhagen J, Mseeh F, Liu Q, Nouraldeen A, Wilson AGE, Kimball SD, Powell DR, Rawlins DB. J Med Chem, 2009, 52: 6201–6204
Goodwin NC, Ding ZM, Harrison BA, Strobel ED, Harris AL, Smith M, Thompson AY, Xiong W, Mseeh F, Bruce DJ, Diaz D, Gopinathan S, Li L, O’Neill E, Thiel M, Wilson AGE, Carson KG, Powell DR, Rawlins DB. J Med Chem, 2017, 60: 710–721
Markham A, Keam SJ. Drugs, 2019, 79: 1023–1029
Cheng G, Fan R, Hernàndez-Torres JM, Boulineau FP, Wei A. Org Lett, 2007, 9: 4849–4852
Bednarski M, Danishefsky S. J Am Chem Soc, 1986, 108: 7060–7067
Zhdanov YA, Alexeev YE, Dorofeenko GN. Carbohydr Res, 1968, 8: 121–122
Saha NN, Desai VN, Dhavale DD. J Org Chem, 1999, 64: 1715–1719
Reddy BVS, Majumder N, Sridhar B. Tetrahedron Lett, 2014, 55: 6081–6084
Zhang F, Xi Y, Lu Y, Wang L, Liu L, Li J, Zhao Y. Chem Commun, 2014, 50: 5771–5773
Mondal RR, Khamarui S, Maiti DK. ACS Omega, 2016, 1: 251–263
Liu H, Liang Y, Jia TT, Han F, Zhang F, Zhao Y. Eur J Org Chem, 2017, 2017(11): 1443–1449
Zhang F, Li Y, Gao F, Liu H, Zhao Y. Carbohydr Res, 2019, 477: 39–50
Vismara E, Torri G, Pastori N, Marchiandi M. Tetrahedron Lett, 1992, 33: 7575–7578
Kuwana D, Ovadia B, Kamimura D, Nagatomo M, Inoue M. Asian J Org Chem, 2019, 8: 1088–1091
For a review on glycosylation with classical glycosyl radicals: Togo H, He W, Waki Y, Yokoyama M. Synlett, 1998, 1998(7): 700–717
For synthesis of classical C-Aryl glycosides with glycosyl radicals: Togo H, Fujii M, Ikuma T, Yokoyama M. Tetrahedron Lett, 1991, 32: 3377–3380
He W, Togo H, Waki Y, Yokoyama M. J Chem Soc Perkin Trans 1, 1998, 2425–2434
Adak L, Kawamura S, Toma G, Takenaka T, Isozaki K, Takaya H, Orita A, Li HC, Shing TKM, Nakamura M. J Am Chem Soc, 2017, 139: 10693–10701
Ma Y, Liu S, Xi Y, Li H, Yang K, Cheng Z, Wang W, Zhang Y. Chem Commun, 2019, 55: 14657–14660
Liu J, Lei C, Gong H. Sci China Chem, 2019, 62: 1492–1496
For recent synthesis of non-classical C-Aryl glycosides with non-classical glycosyl radicals: Zhou X, Wang P, Zhang L, Chen P, Ma M, Song N, Ren S, Li M. J Org Chem, 2018, 83: 588–603
Wang Y, Yang L, Liu S, Huang L, Liu Z-. Adv Synth Catal, 2019, 361: 4568–4574
Dumoulin A, Matsui JK, Gutiérrez-Bonet Á, Molander GA. Angew Chem Int Ed, 2018, 57: 6614–6618
For reviews on hydrogenation using 1,4-dihydropyridine as hydride donor, see: Ouellet SG, Walji AM, MacMillan DWC. Acc Chem Res, 2007, 40: 1327–1339
Zheng C, You SL. Chem Soc Rev, 2012, 41: 2498–2518
For recent reviews on alkylation reaction using 4-alkyl-1,4-dihy-dropyridine as alkyl donor: Huang W, Cheng X. Synlett, 2016, 28: 148–158
Ye S, Wu J. Acta Chim Sin, 2019, 77: 814–831
For early examples on dealkylative aromatization of 4-alkyl-1,4-dihydropyridines: Loev B, Snader KM. J Org Chem, 1965, 30: 1914–1916
Lee JS, Jacobsen NE, Ortiz de Montellano PR. Biochemistry, 1988, 27: 7703–7710
Zhang D, Wu LZ, Zhou L, Han X, Yang QZ, Zhang LP, Tung CH. J Am Chem Soc, 2004, 126: 3440–3441
Fang X, Liu YC, Li C. J Org Chem, 2007, 72: 8608–8610
For the synthesis of 4-alkyl-1,4-dihydropyridines: Tewari N, Dwivedi N, Tripathi RP. Tetrahedron Lett, 2004, 45: 9011–9014
Lu L, Xu H, Zhou P, Yu F. Chin J Org Chem, 2016, 36: 2858–2879
Li G, Chen R, Wu L, Fu Q, Zhang X, Tang Z. Angew Chem Int Ed, 2013, 52: 8432–8436
Nakajima K, Nojima S, Nishibayashi Y. Angew Chem Int Ed, 2016, 55: 14106–14110
Nakajima K, Nojima S, Sakata K, Nishibayashi Y. ChemCatChem, 2016, 8: 1028–1032
Buzzetti L, Prieto A, Roy SR, Melchiorre P. Angew Chem Int Ed, 2017, 56: 15039–15043
Chen W, Liu Z, Tian J, Li J, Ma J, Cheng X, Li G. J Am Chem Soc, 2016, 138: 12312–12315
Li G, Wu L, Lv G, Liu H, Fu Q, Zhang X, Tang Z. Chem Commun, 2014, 50: 6246–6248
McDonald BR, Scheidt KA. OrgLett, 2018, 20: 6877–6881
Nakajima K, Guo X, Nishibayashi Y. Chem Asian J, 2018, 13: 3653–3657
Verrier C, Alandini N, Pezzetta C, Moliterno M, Buzzetti L, Hepburn HB, Vega-Peñaloza A, Silvi M, Melchiorre P. ACS Catal, 2018, 8: 1062–1066
van Leeuwen T, Buzzetti L, Perego LA, Melchiorre P. Angew Chem Int Ed, 2019, 58: 4953–4957
Liu X, Liu R, Dai J, Cheng X, Li G. Org Lett, 2018, 20: 6906–6909
Song ZY, Zhang CL, Ye S. Org Biomol Chem, 2018, 17: 181–185
Chen X, Ye F, Luo X, Liu X, Zhao J, Wang S, Zhou Q, Chen G, Wang P. J Am Chem Soc, 2019, 141: 18230–18237
Liang S, Kumon T, Angnes RA, Sanchez M, Xu B, Hammond GB. Org Lett, 2019, 21: 3848–3854
Nakajima K, Zhang Y, Nishibayashi Y. OrgLett, 2019, 21: 4642–4645
Xie S, Li D, Huang H, Zhang F, Chen Y. J Am Chem Soc, 2019, 141: 16237–16242
For enantioselective alkylation with 4-alkyl-1,4-dihydropyridines: de Assis FF, Huang X, Akiyama M, Pilli RA, Meggers E. J Org Chem, 2018, 83: 10922–10932
Zhang HH, Zhao JJ, Yu S. J Am Chem Soc, 2018, 140: 16914–16919
Zhang K, Lu LQ, Jia Y, Wang Y, Lu FD, Pan F, Xiao WJ. Angew Chem Int Ed, 2019, 58: 13375–13379
Gandolfo E, Tang X, Raha Roy S, Melchiorre P. Angew Chem Int Ed, 2019, 58: 16854–16858
For acylation reactions with 4-acyl-DHPs: Bieszczad B, Perego LA, Melchiorre P. Angew Chem Int Ed, 2019, 58: 16878–16883
Goti G, Bieszczad B, Vega-Peñaloza A, Melchiorre P. Angew Chem Int Ed, 2019, 58: 1213–1217
Gutiérrez-Bonet Á, Tellis JC, Matsui JK, Vara BA, Molander GA. ACS Catal, 2016, 6: 8004–8008
Gutiérrez-Bonet Á, Remeur C, Matsui JK, Molander GA. J Am Chem Soc, 2017, 139: 12251–12258
Badir SO, Dumoulin A, Matsui JK, Molander GA. Angew Chem Int Ed, 2018, 57: 6610–6613
Phelan JP, Lang SB, Sim J, Berritt S, Peat AJ, Billings K, Fan L, Molander GA. J Am Chem Soc, 2019, 141: 3723–3732
For selected reviews on C-nucleosides: Ferrero M, Gotor V. Chem Rev, 2000, 100: 4319–4348
Wu Q, Simons C. Synthesis, 2004, 2004(10): 1533
Bokor É, Kun S, Goyard D, Tóth M, Praly JP, Vidal S, Somsàk L. Chem Rev, 2017, 117: 1687–1764
Stambaský J, Hocek M, Kocovský P. Chem Rev, 2009, 109: 6729–6764
De Clercq E. J Med Chem, 2016, 59: 2301–2311
Temburnikar K, Seley-Radtke KL. Beilstein J Org Chem, 2018, 14: 772–785
Shiraishi T, Kuzuyama T. J Antibiot, 2019, 72: 913–923
The stereochemistry was assigned via 2-D 1H NMR analysis (See Supporting Information for details)
We believe the stereoselectivity of this Minisci-type alkylation reaction originated from a combined effect of stereoelectronic and steric interactions. Anomeric effect and quasi-anomeric stabilization make the anomeric radical prefer to adopt axial conformation, and the N-heterocycles would approach the radical intermediates from less hindered face (See Supporting Information for details)
Gu F, Huang W, Liu X, Chen W, Cheng X. Adv Synth Catal, 2018, 360: 925–931
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21672105, 21725204, 91753124), Natural Science Foundation of Tianjin (17JCYBJC19700, 18JCZDJC32800), and the Fundamental Research Funds for the Central Universities (Nankai University) (63191746).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supporting information
The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
11426_2020_9813_MOESM1_ESM.pdf
Synthesis of Non-classical Heteroaryl C-Glycosides via Minisci-type Alkylation of N-Heteroarenes with 4-Glycosyl-dihydropyridines
Rights and permissions
About this article
Cite this article
Wang, Q., Duan, J., Tang, P. et al. Synthesis of non-classical heteroaryl C-glycosides via Minisci-type alkylation of N-heteroarenes with 4-glycosyl-dihydropyridines. Sci. China Chem. 63, 1613–1618 (2020). https://doi.org/10.1007/s11426-020-9813-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-020-9813-5