Abstract
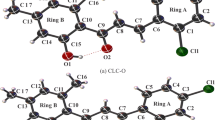
Short chain chlorinated paraffins (SCCPs) are not only research focus of environmental issues but also interesting model molecules for organic chemistry which exhibit diverse conformation preference and intramolecular noncovalent interactions (NCIs). A systematic study was conducted to reveal the conformation preference and the related intramolecular NCIs in two C10-isomers of SCCPs, 5,5,6,6-tetrachlorodecane and 4,4,6,6-tetrachlorodecane. The overall conformation profile was determined on the basis of relative energies calculated at the MP2/6-311++G(d,p) level with the geometries optimized by B3LYP/6-311++G(d,p) method. Then, quantum theory of atoms in molecules (QTAIM) has been adopted to identify the NCIs in the selected conformers of the model molecules at both B3LYP/6-311++G(d,p) and M06-2X/aug-cc-pvdz level. Different chlorine substitution modes result in varied conformation preference. No obvious gauche effect can be observed for the SCCPs with chlorination on adjacent carbon atoms. The most stable conformer of 5,5,6,6-tetrachlorodecane (tTt) has its three dihedral angles in the T configuration, and there is no intramolecular NCIs found in this molecule. On the contrary, the chlorination on interval carbon atoms favors the adoption of gauche configuration for the H–C–C–Cl axis. Not only intramolecular H···Cl contacts but also H···H interactions have been identified as driving forces to compensate the instability from steric crowding of the gauche configuration. The gggg and g′g′g′g′ conformers are the most popular ones, while the populations of tggg and tg′g′g′ conformer are second to those of the gggg and g′g′g′g′ conformers. Meanwhile, the M06-2X method with large basis sets is preferred for identification of subtle intramolecular NCIs in large molecules like SCCPs.
Similar content being viewed by others
References
Tomy GT, Stern GA, Lockhart WL, Muir DCG. Environ Sci Technol, 1999, 33: 2858–2863
Tomy GT, Fisk AT, Westmore JB, Muir DCG. Environmental chemistry and toxicology of polychlorinated n-alkanes. In: Ware GW, Ed. Reviews of Environmental Contamination and Toxicology. Volume 158. New York: Springer-Verlag, 1998. 53–128
Fiedler H. Short-chain chlorinated paraffins: production, use and international regulations. In: Boer J, Ed. The Handbook of Environmental Chemistry. Volume 10. Chlorinated Paraffins. Berlin/Heidelberg: Springer-Verlag, 2010. 1–40
Sverko E, Tomy GT, Märvin CH, Muir DCG. Environ Sci Technol, 2012, 46: 4697–4698
Wang T, Wang YW, Jiang GB. Environ Sci Technol, 2013, 47: 11924–11925
Chen MY, Luo XJ, Zhang XL, He MJ, Chen SJ, Mai BX. Environ Sci Technol, 2011, 45: 9936–9943
Geng NB, Zhang HJ, Zhang BQ, Wu P, Wang FD, Yu ZK, Chen JP. Environ Sci Technol, 2015, 49: 3076–3083
Drouillard KG, Tomy GT, Muir DCG, Friesen KJ. Environ Toxicol Chem, 1998, 17: 1252–1260
Muir DCG, Stern G, Tomy G. Chlorinated paraffins. In: Hutzinger O, Paasivirta J, Eds. The Handbook of Environmental Chemistry. Volume 3. Anthropogenic Compounds Part K. New Types of Persistent Halogenated Compounds. Berlin/Heidelberg: Springer-Verlag, 2000. 203–236
Ma XD, Zhang HJ, Zhou HQ, Na GS, Wang Z, Chen C, Chen JW, Chen JP. Atmos Environ, 2014, 90: 10–15
http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/sccps.ht ml, 2015-04-27
Glüge J, Bogdal C, Scheringer M, Buser AM, Hungerbühler K. J Phys Chem Ref Data, 2013, 42: 023103
Li C, Xie HB, Chen JW, Yang XH, Zhang YF, Qiao XL. Environ Sci Technol, 2014, 48: 13808–13816
Müller-Dethlefs K, Hobza P. Chem Rev, 2000, 100: 143–168
Aoki M, Ohashi Y, Masuda S, Ojima S, Ueno N. J Chem Phys, 2005, 122: 194508
Vetter AJ, Rieth RD, Brennessel WW, Jones WD. J Am Chem Soc, 2009, 131: 10742–10752
Takahashi O, Kohno Y, Nishio M. Chem Rev, 2010, 110: 6049–6076
Morino Y, Kuchitsu K. J Chem Phys, 1958, 28: 175–184
Hirota E. J Chem Phys, 1962, 37: 283–291
Monteiro NKV, Firme CL. J Phys Chem A, 2014, 118: 1730–1740
Johansson MP, Swart M. Phys Chem Chem Phys, 2013, 15: 11543–11553
Bader RFW. Chem Rev, 1991, 91: 893–928
Koch U, Popelier PLA. J Phys Chem, 1995, 99: 9747–9754
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang WT. J Am Chem Soc, 2010, 132: 6498–6506
Lopes Jesus AJ, Rosado MT, Reva I, Fausto R, Eusébio ME, Redinha JS. J Phys Chem A, 2006, 110: 4169–4179
Zhao Y, Truhlar DG. J Chem Theory Comput, 2006, 2: 1009–1018
Jablonski M. J Phys Chem A, 2012, 116: 3753–3764
Forni A. J Phys Chem A, 2009, 113: 3403–3412
Wodrich MD, Corminboeuf C, Schleyer PvR. Org Lett, 2006, 8: 3631–3634
Schreiner PR, Fokin AA, Pascal RA, de Meijere A. Org Lett, 2006, 8: 3635–3638
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09. Revision B.01. Wallingford, CT: Gaussian, Inc., 2009
Liu RR, Zhang CX, Kang LY, Sun XM, Zhao Y. RSC Adv, 2015, 5: 37988–37994
Colebourne N, Stern ES. J Chem Soc, 1965: 3599–3605
Biegler-König F, Schönbohm J. J Comput Chem, 2002, 23: 1489–1494
Jin R, Sun W. Sci China Chem, 2012, 55: 1428–1434
Zhao Y, Truhlar DG. Acc Chem Res, 2008, 41: 157–167
Yan XQ, Zhao XR, Wang H, Jin WJ. J Phys Chem B, 2014, 118: 1080–1087
Laurent A, Jacquemin D. Sci China Chem, 2014, 57: 1363–1368
Walker M, Harvey A, Sen A, Dessent C. J Phys Chem A, 2013, 117: 12590–12600
Cerón-Carrasco J, Jacquemin D, Graton J, Thany S, Questel JY. J Phys Chem A, 2013, 117: 3944–3953
Wladkowski BD, Broadwater SJ. J Chem Edu, 2002, 79: 230–233
Thomas TD, Sæthre LJ, Børve KJ. Phys Chem Chem Phys, 2007, 9: 719–724
Pan WX, Zhang DJ, Han Z, Zhan JH, Liu CB. Environ Sci Technol, 2013, 47: 8489–8498
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Y., Pan, W., Fu, J. et al. Conformation preference and related intramolecular noncovalent interaction of selected short chain chlorinated paraffins. Sci. China Chem. 59, 338–349 (2016). https://doi.org/10.1007/s11426-015-5502-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-015-5502-y