Abstract
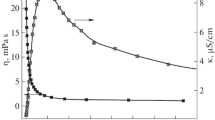
Studies of the density and the excess molar volume of ethylene glycol (EG)-water mixtures were carried out to illustrate the hydrogen bonding interactions of EG with water at different temperatures. The results suggest that a likely complex of 3 ethylene glycol molecules bonding with 4 water molecules in an ethylene glycol-water mixture (EGW) is formed at the maximal excess molar volume, which displays stronger absorption capabilities for SO2 when the concentration of SO2 reaches 400×106 (volume ratio) in the gas phase. Meanwhile, FTIR and UV spectra of EGWs were recorded at various EG concentrations to display the hydrogen bonding interactions of EG with water. The FTIR spectra show that the stretching vibrational band of hydroxyl in the EGWs shifts to a lower frequency and the bending vibrational band of water shifts to a higher frequency with increasing the EG concentration, respectively. Furthermore, the UV spectra show that the electron transferring band of the hydroxyl oxygen in EG shows red shift with increasing the EG concentration. The frequency shifts in FTIR spectra and the shifts of absorption bands in UV absorption spectra of EGWs are interpreted as the strong hydrogen bonding interactions of the hydrogen atoms in water with the hydroxyl oxygen atoms of EG.
Similar content being viewed by others
References
Lasgabaster A, Abad M J, Barral L, Ares A. FTIR study on the nature of water sorbed in polypropylene (PP)/ethylene alcohol vinyl (EVOH) films. Eur Polym J, 2006, 42: 3121–3132
Dharmalingam K, Ramachandran K P. FTIR and dielectric studies of molecular interaction between alkyl methacrylates and primary alcohols. Physica B, 2006, 4: 1–5
Palombo F, Paolantoni M, Sassi P, Morresi A, Cataliotti R S. Spectroscopic studies of the “free” OH stretching bands in liquid alcohols. J Mol Liq, 2006, 125: 139–146
Yuan B, Dou X M. Near-infrared spectral studies of hydrogen-bond in water-methanol mixtures. Spectrosc Spect Anal (in Chinese), 2004, 11: 1319–1322
Desulphurization in coal combustion systems. The Institution of Chemical Engineering. 1989
Li X X, Liu Y X, Wei X H. Hydrolysis of carbonyl sulfide in binary mixture of diethylene glycol diethyl ether and water. Chinese J Chem Eng, 2005, 13(2): 234–238
Wei X H. Desulfurication & decarburization solution activities. CN. 02130605. 2. 2002
Potteau E, Levillain E, Lelieur J P. Mechanism of the electrochemical reduction of sulfur dioxide in non-aqueous solvents. J Electroanal Chem, 1999, 476: 15–25
van Dam M H H, Lamine A S, Roizard D, Lochon P, Roizard. Selective sulfur dioxide removal using organic solvents. Ind Eng Chem Res, 1997, 36: 4628–4637
Ivopoulos P, Sotiropoulou M, Bokias G, Staikos G. Water-soluble hydrogen-bonding interpolymer complex formation between poly (ethylene glycol) and poly(acrylic acid) grafted with poly(2-acrylamido-2-methylpropanesulfonic acid). Langmuir, 2006, 22: 9181–9186
Schofield D P, Lane J R, Kjaergaard H G. Hydrogen bonded OH-stretching vibration in the water dimer. J Phys Chem A, 2007, 111: 567–572
Errington J R, Boulougouris G C, Economou I G, Panagiotopoulos A Z, Theodorou D N. Molecular simulation of phase equilibria for water-methane and water-ethane mixtures. J Phys Chem B, 1998, 102: 8865–8873
Dashnau J L, Nucci N V, Sharp K A. Hydrogen bonding and the cryoprotective properties of glycerol/water mixtures. J Phys Chem B, 2006, 110: 13670–13677
Nose A, Hamsaki T, Hojo M, Kato R, Uetara K, Ueda T. Hydrogen bonding in alcoholic beverages (distilled spirits) and water-ethanol mixtures. J Agric Food Chem, 2005, 53: 7074–7081
Tong J B, Liu S L, Li M P, Zhang S W. Study on the spectrum of ethanol-water association system. Liq Sci Tech (in Chinese), 2003, 3: 83–84
Tsierkezos N G, Molinou I E. Densities and viscosities of ethylene glycol binary mixtures at 293.15 K. J Chem Eng Data, 1999, 44: 955–958
Naidu B V K, Rao K C, Subha M C S. Densities and viscosities of mixtures of some glycols and polyglycols in dimethyl sulfoxide at 308.15 K. J Chem Eng Data, 2002, 47: 379–382
Pal A, Sharma S. Excess molar volumes and viscosities of binary liquid mixtures of ethylene glycol diethyl ether + ethylene glycol monomethyl, + diethylene glycol monomethyl, + triethylene glycol monomethyl ethers at 298.15 and 308.15 K. J Chem Eng Data, 1999, 44: 1067–1070
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Yongfeng Boyuan Industry Co., Ltd. (Jiangxi Province, China)
Rights and permissions
About this article
Cite this article
Zhang, J., Zhang, P., Ma, K. et al. Hydrogen bonding interactions between ethylene glycol and water: density, excess molar volume, and spectral study. Sci. China Ser. B-Chem. 51, 420–426 (2008). https://doi.org/10.1007/s11426-008-0045-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-008-0045-0