Abstract
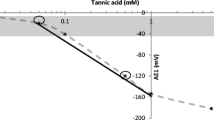
It is difficult to describe the taste of Processed Aconite Root (PAR) because it contains toxic compounds, and tasting poses some risk to the examiner. Therefore, there is no description of the taste of PAR in the latest Japanese Pharmacopoeia, although the taste of crude drugs has been regulated as a criterion for judgment. In this study, we revealed the objective taste of PAR by using a taste-sensing system. The PAR samples examined were classified into four types by how the samples were processed: PAR1 processed by autoclaving; PAR2-a processed by autoclaving after rinsing in salt (sodium chloride) solution; PAR2-h processed by heating after rinsing in calcium chloride solution; PAR3 processed by treating with hydrated lime after rinsing in salt solution. The most characteristic taste factor of PAR is an aftertaste of cationic bitterness, which was detected in all PAR sample solutions, even at the concentration of 0.1 mg/ml. In addition, anionic bitterness and saltiness were detected in all sample solutions at 1 mg/ml. Furthermore, umami was detected in the PAR1, PAR2-a, and PAR3 sample solutions at 1 mg/ml. Detailing the analyses of the four taste factors on the four sample types, we found each type has its own characteristic taste pattern. On the basis of these results, we proposed a method for discriminating one PAR type from another by using the system.





Similar content being viewed by others
References
Matsumoto K (ed) (1984) Shinkoku-koho Shinnohonzokyo. Shobundo, Tokyo, pp 185–186
Hikino H, Yamada C, Nakamura K, Sato H, Ohizumi Y, Endo K (1977) Change of alkaloid composition and acute toxicity of Aconitum roots during processing. Yakugaku Zasshi 97:359–366
Kosuge T, Yokota M (1976) Studies on cardiac principle of aconite root. Chem Pharm Bull 24:176–178
Higashi K (1996) A case of postherpetic neuralgia successfuly controlled with 3 Kampo medicines which Bushi commonly. Jpn J Orient Med 47:267–270
Toriizuka K (ed) (2003) Monographs of pharmacological research on traditional herbal medicines. Ishiyaku Publishers, Inc., Tokyo, pp 401–413
Nakae H (2009) Efficacy of aconite tuber powder in patients with arthralgia and somatic pain. Kampo Med 60:81–85
Chino A, Ishida A, Sekiya N, Ohno K, Hirasaki Y, Kasahara Y, Namiki T, Miyazaki M, Terasawa K (2010) A case of multiple intractable skin ulcers of bilateral legs due to arteriovenous fistula successfully treated with Kampo medicines. Kampo Med 61:325–330
The Ministry of Health and Welfare Notification No. 65, March 15, 1955
The Ministry of Health and Welfare Notification No. 76, April 1, 1961
The Ministry of Health and Welfare Notification No. 163, April 1, 1966
Hikino H, Shiota S, Takahashi M, Murakami M (1983) Seasonal dynamics of the accumulation of aconite alkaloids in Aconitum carmichaeli roots. Jpn J Pharmacog 37:68–72
Kitagawa I, Chen ZL, Yoshihara M, Yoshikawa M (1984) Chemical studies on crude drug processing. IV. Aconiti Tuber (3), Quantitative determination of aconitine alkaloids in aconiti tuber by means of high performance liquid chromatography. Yakugaku Zasshi 104:867–872
Mori T, Murayama M, Bando H, Kawahara N (1991) Studies on the constituents of Aconitum species. XII. Syntheses of Jesaconitine derivatives and their analgestic and toxic activities. Chem Pharm Bull 39:379–383
Taki M, Omiya Y, Suzuki Y, Ikeda Y, Noguchi M, Matuba T, Kubo M, Niitu K, Komatsu Y, Okada M (1998) Quality and pharmacological investigation of processed aconiti tuber (TJ-3022). Nat Med 52:343–352
Nose M, Arai T, Zhao CH, Kojima K, Ogihara Y, Sekita S, Satake M (2001) Quantitative determination of aconitine alkaloids in aconiti tuber and Kampo prescription containing aconiti tuber commercially available. Nat Med 55:124–133
Taki M, Terabayashi S, Matsuba T, Sasaki H, Fukuchi M, Okada M (2002) Quality investigation of aconiti tuber in China and Japan. Nat Med 56:163–172
Okada K, Kawaguchi K (2004) The effect of tuberous root size on growth and alkaloid content of aconite (Aconitum subcuneatum). Nat Med 58:49–54
Taki M, Matsuba T, Fukuchi M, Aburada M, Okada M (2004) Comparison of seasonal variations on growth of Aconitum carmichaeli DEBX. and constituents of root tubers cultivated in Hokkaido and Ibaraki prefecture. Nat Med 58:55–63
Okada K, Kawaguchi K (2005) Change in chemical component characters within and among years of aconite. Nat Med 59:36–41
Nakamura Y, Yomura K, Kammoto T, Ishimatsu M, Kikuchi Y, Niitsu K, Terabayashi S, Takeda S, Sasaki H, Arimoto K, Okada M, Sekita S, Satake M, Goda Y (2006) Physicochemical quality evaluation of natural compounds isolated from crude drugs. Standard compounds for the official specification and testing method of “Processed Aconite Root” and “Powdered Processed Aconite Root” in the Japanese Pharmacopoeia. J Nat Med 60:285–294. doi:10.1007/s11418-006-0005-y
The Ministry of Health, Labour and Welfare Ministerial Notification No. 461, December 28, 2004. http://jpdb.nihs.go.jp/jp14supp2/YAK2T.pdf
Sato M, Anetai M, Goda Y (2005) Analysis of organophosphorus pesticide residues in crude drugs. Pharm Regul Sci 36:83–97
Yamamoto K, Yamamoto T, Kondo S, Tamura M, Shibata Y, Umeda K, Akiba S, Kawakami T, Saito F, Sugimoto T, Isomi Y, Nakada T, Takao M, Nakashima K, Tahara M, Hayashi K, Sudo M, Nakanishi K, Isozaki O, Kawahara N, Goda Y (2005) Assay of ginsenoside Rg1 and ginsenoside Rb1 in ginseng and red ginseng by high-performance liquid chromatography. Pharm Regul Sci 36:211–222
Kawahara N, Kim IH, Goda Y (2006) Content of sulfur dioxides in herbal materials obtained form the Japanese market. Jpn J Food Chem 13:105–108
Sato M, Anetai M, Goda Y (2006) Organophosphorus pesticide residues in decoctions of crude drugs. Pharm Regul Sci 37:245–250
Kawahara N, Anjiki N, Kim IH, Mikage M, Goda Y (2007) Studies on the relationship between color and content of sulfur dioxides in crude drugs obtained from the Japanese market. Jpn J Food Chem 14:140–144
Maruyama T, Sugimoto N, Kuroyanagi M, Kim IH, Kamakura H, Kawasaki T, Fujita M, Shimada H, Yamamoto Y, Tada A, Yamazaki T, Goda Y (2007) Authentication and chemical study of Isodonis Herba and Isodonis extracts. Chem Pharm Bull 55:1626–1630
Maruyama T, Kamakura H, Miyai M, Komatsu K, Kawasaki T, Fujita M, Shimada H, Yamamoto Y, Goda Y (2008) Authentication of the traditional medicinal plant Eleutherococcus senticosus by DNA and chemical analyses. Planta Med 74:787–789. doi:10.1055/s-2008-1074537
Sato M, Anetai M, Kamakura H, Goda Y (2008) Analysis of organophosphorus pesticide residues in crude drugs (Part 2). Pharm Regul Sci 39:203–222
Tokumoto H, Shimomura Y, Katsuki S, Goda Y (2008) Morphological discrimination of Curcuma longa L. and Curcuma aromatica Salisb. Jpn J Pharmacog 62:54–65
Goda Y, Kawahara N, Kiuchi F, Hirakura K, Kikuchi Y, Nishimura H, Marumoto M, Kitazaki H (2009) A guanidine derivative from seeds of Plantago asiatica. J Nat Med 63:58–60. doi:10.1007/s11418-008-0275-7
Kawahara N, Anjiki N, Hosoe J, Kim IH, Ikezaki H, Mikage M, Goda Y (2009) Studies on relationship between taste and content of sulfur dioxide in crude drugs obtained from the Japanese market. Pharm Regul Sci 40:129–135
Kondo K, Shiba M, Yotsuyanagi Y, Nishimura N, Maruyama T, Goda Y (2009) Discrimination between Atractylodes Rhizome (Byaku-jutsu) and Atractylodes lancea Rhizome (So-jutsu) by the PCR-RFLP analysis of ITS region on nrDNA. J Jpn Bot 84:356–359
Terabayashi S, Sakai E, Yamaji H, Kondo K, Kawahara N, Goda Y (2009) Authentication and standardization of botanical origin and morphology of Coix Fruit in the Japanese Pharmacopoeia. J Jpn Bot 84:77–84
Maruyama T, Miyai M, Kamakura H, Nakajima I, Kawasaki T, Komatsu K, Fujita M, Yamamoto Y, Shibata T, Goda Y (2010) The authentication and the purity test of Eleutherococcus Senticosus Rhizome based on the genetic approach. Jpn J Pharmacog 64:15–20
Sato M, Anetai M, Kamakura H, Goda Y (2010) Analysis of organophosphorus pesticide residues in crude drugs (Part 3). Pharm Med Dev Regul Sci 41:324–337
Maruyama T, Kondo K, Yotsuyanagi Y, Yamamoto Y, Kawasaki T, Shiba M, Terasaka K, Yamane M, Zhu S, Sakata K, Fujita M, Akiyama H, Nishimura N, Komatsu K, Mizukami H, Goda Y (2010) The inter-laboratory validation study for the purity test of crude drugs based on a PCR-RFLP. Jpn J Pharmacog 64:96–101
Anjiki N, Kawahara N, Goda Y (2005) Evaluation of the taste of Kampo formulae by taste-sensing system (1). Nat Med 59:164–170
Anjiki N, Suzuki A, Kawahara N, Goda Y (2006) Evaluation of the taste of a Kampo formula by a taste-sensing system (2), taste of Kakkonto. Jpn J Pharmacog 60:21–27
Anjiki N, Yoshino C, Kawahara N, Goda Y (2007) Evaluation of the taste of a Kampo formula by a taste-sensing system (3), the taste of Ryokeijutsukanto. Jpn J Pharmacog 61:6–13
The Ministry of Health, Labour and Welfare Ministerial Notification No. 285, March 31, 2006. http://jpdb.nihs.go.jp/jp15/YAKKYOKUHOU15.pdf
Toko K (2000) Biomimetic sensor technology. The Press Syndicate of The University of Cambridge, Cambridge
Toko K, Uchida T (2007) Taste modification technology of food and medicine. CMC Publishing Co., Ltd., Tokyo, pp 219–252
Habara M, Toko K (2009) Biomimetic membrane for taste sensing, Chap. 6. In: Ariga K, Nalwa HS (eds) Bottom-up nanofabrication, vol 6. American Scientific Publishers, Los Angeles, pp 91–109
Ikezaki H, Taniguchi A, Toko K (1998) Increase in information by improvement of measuring method in a multichannel taste sensor. TIEE Japan 118-E:506–512
Pfaffmann C (1959) Neurophysiology. In: Field J (ed) Handbook of physiology, vol 1. American Physiological Society, Washington, DC, pp 507–534
Schutz HG, Pilgrim ES (1957) Differential sensitivity in gustation. J Exp Psychol 54:41–48
Bai G, Yang Y, Shi Q, Liu Z, Zhang Q, Zhu YY (2008) Identification of higenamine in radix aconiti Lateralis Preparata as a beta2-adrenergic receptor agonist. Acta Pharmacol Sin 29:1187–1194
Matsui M, Bando H, Murayama M, Miura T (1999) Constituent of “KAKO-BUSHI-MATSU” II Components of amino acid and sugars. Nat Med 53:313–315
Ohnishi S (1975) A spin-label study of biological membranes with special emphasis on calcium-induced lateral phase separation. Adv Biophys 8:35–82
Acknowledgements
The authors are grateful to members of PAR-WG for kindly providing PAR samples and information for aconite processing. This work was supported in part by a Health and Labour Sciences Research Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anjiki, N., Hosoe, J., Fuchino, H. et al. Evaluation of the taste of crude drug and Kampo formula by a taste-sensing system (4): taste of Processed Aconite Root. J Nat Med 65, 293–300 (2011). https://doi.org/10.1007/s11418-010-0489-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-010-0489-3