Abstract
Purpose
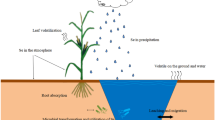
Soil selenium (Se) sequestration and transformation, which are strongly controlled by soil redox conditions, are critical for understanding the mobility and bioavailability in the environment. Thus, the effect of redox potential on Se transformation was investigated for exploring the release mechanism of Se in soil.
Materials and methods
Soils were incubated under anoxic condition in four treatments at room temperature over 56 days, and the soil solution pH, Eh, and Fe and Se concentrations were measured at given reaction time. The sequential extraction and X-ray photoelectron spectroscopy (XPS) were used to obtain the species distribution of Se in soil. High-resolution transmission electron microscopy (HR-TEM) was employed to observe morphology characteristic of soil.
Results and discussion
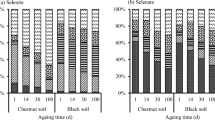
Parts of soil Se can be released into solution, and Se speciation in soil changed during the incubation period. XPS and sequential extraction analyses revealed that the primary speciation of Se in soil was elemental Se, and metallic selenides were formed under aerobic condition. Moreover, XPS and HR-TEM data revealed the crystalline state of iron oxides in soil changed after anoxic incubation, and certain amorphous iron oxides were formed.
Conclusions
Se release is activated by short-term incubation, whereas Se can be transformed into less soluble state after long-term incubation. Organic matter takes extremely an important role in Fe oxide reductive dissolution and Se transformation. This study is useful to understand the environmental behaviors of Se and enhance the application of Se fertilizers effectively and safely in Se deficiency area.







Similar content being viewed by others
References
Abdel-Samad H, Watson PR (1997) An XPS study of the adsorption of chromate on goethite (α-FeOOH). Appl Surf Sci 108:371–377
Ashworth DJ, Moore J, Shaw G (2008) Effects of soil type, moisture content, redox potential and methyl bromide fumigation on Kd values of radio-selenium in soil. J Environ Radioact 99:1136–1142
Chakraborty S, Bardelli F, Charlet L (2010) Reactivities of Fe(II) on calcite: selenium reduction. Environ Sci Technol 44:1288–1294
Combs GF (2007) Selenium in global food systems. Brit J Nutr 85:517–547
Dhillon KS, Dhillon SK (1999) Adsorption-desorption reactions of selenium in some soils of India. Geoderma 93:19–31
Eswayah AS, Smith TJ, Gardiner PHE (2016) Microbial transformations of selenium species of relevance to bioremediation. Appl Environ Microbiol 82:4848–4859
Eswayah AS, Smith TJ, Scheinost AC, Hondow N, Gardiner PHE (2017) Microbial transformations of selenite by methane-oxidizing bacteria. Appl Microbiol Biotechnol 101:6713–6724
Fan JX, Wang YJ, Liu C, Wang LH, Yang K, Zhou DM, Li W, Sparks DL (2014) Effect of iron oxide reductive dissolution on the transformation and immobilization of arsenic in soils: new insights from X-ray photoelectron and X-ray absorption spectroscopy. J Hazard Mater 279:212–219
Fan J-X, Wang Y-J, Fan T-T, Dang F, Zhou D-M (2016) Effect of aqueous Fe(II) on Sb(V) sorption on soil and goethite. Chemosphere 147:44–51
Février L, Martin-Garin A, Leclerc E (2007) Variation of the distribution coefficient (Kd) of selenium in soils under various microbial states. J Environ Radioact 97:189–205
Grosvenor AP, Kobe BA, Biesinger MC, McIntyre NS (2004) Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf Interface Anal 36:1564–1574
Hamdadou N, Bernède JC, Khelil A (2002) Preparation of iron selenide films by selenization technique. J Cryst Growth 241:313–319
Han DS, Batchelor B, Abdel-Wahab A (2013) XPS analysis of sorption of selenium(IV) and selenium(VI) to mackinawite (FeS). Environ Prog Sustain 32:84–93
Hibbs BJ, Lee M, Ridgway R (2017) Land use modification and changing redox conditions releases selenium and sulfur from historic marsh sediments. J Contemp Water Res Edu 161:48–65
Hingston FJ, Posner AM, Quirk JP (1968) Adsorption of selenite by goethite, adsorption from aqueous solution. Am Chem Soc:82–90
Jensen MB, Hansen HCB, Nielsen NE, Magid J (1998) Phosphate mobilization and immobilization in two soils incubated under simulated reducing conditions. Acta Agric Scand Sect B Soil Plant Sci 48:11–17
Kulp TR, Pratt LM (2004) Speciation and weathering of selenium in upper cretaceous chalk and shale from South Dakota and Wyoming, USA. Geochim Cosmochim Acta 68:3687–3701
Lessa JHL, Araujo AM, Silva GNT, Guilherme LRG, Lopes G (2016) Adsorption-desorption reactions of selenium (VI) in tropical cultivated and uncultivated soils under Cerrado biome. Chemosphere 164:271–277
Manceau A, Charlet L (1994) The mechanism of selenate adsorption on goethite and hydrous ferric oxide. J Colloid Interf Sci 168:87–93
Masscheleyn PH, Patrick WH (1993) Biogeochemical processes affecting selenium cycling in wetlands. Environ Toxicol Chem 12:2235–2243
Medicine, F.a.N.B.-U.I.o (2000) Dietary references intakes for vitamin C, vitamin E, selenium and carotenoids. National Academy Press, Washington
Myneni SCB, Tokunaga TK, Brown GE (1997) Abiotic selenium redox transformations in the presence of Fe(II,III) oxides. Science 278:1106–1109
Nancharaiah YV, Lens PNL (2015) Ecology and biotechnology of selenium-respiring bacteria. Microbiol Mol Biol Rev 79:61–80
Naveau A, Monteil-Rivera F, Guillon E, Dumonceau J (2007) Interactions of aqueous aelenium (−II) and (IV) with metallic sulfide surfaces. Environ Sci Technol 41:5376–5382
NIST X-ray photoelectron spectroscopy database, NIST standard reference database 20, version https://srdata.nist.gov/xps/Default.aspx
Norvell WA, Lindsay WL (1982a) Effect of ferric-chloride additions on the solubility of ferric ion in a near-neutral soil. J Plant Nutr 5:1285–1295
Norvell WA, Lindsay WL (1982b) Estimation of the concentration of estimation of the concentration of Fe3+ and the (Fe3+)(OH−)3 ion product from equilibria EDTA in soil. Soil Sci Soc Am J 46:710–715
Rayman MP (2008) Food-chain selenium and human health: emphasis on intake. Br J Nutr 100:254–268
Roosendaal SJ, van Asselen B, Elsenaar JW, Vredenberg AM, Habraken FHPM (1999) The oxidation state of Fe(100) after initial oxidation in O2. Surf Sci 442:329–337
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Ruby C, Humbert B, Fusy J (2000) Surface and interface properties of epitaxial iron oxide thin films deposited on MgO(001) studied by XPS and Raman spectroscopy. Surf Interface Anal 29:377–380
Sathe SK, Mason AC, Rodibaugh R, Weaver CM (1992) Chemical form of selenium in soybean (Glycine max L.) lectin. J Agric Food Chem 40:2084–2091
Scheinost AC, Charlet L (2008) Selenite reduction by mackinawite, magnetite and siderite: XAS characterization of nanosized redox products. Environ Sci Technol 42:1984–1989
Schulz-Zunkel C, Rinklebe J, Bork H-R (2015) Trace element release patterns from three floodplain soils under simulated oxidized–reduced cycles. Ecol Eng 83:485–495
Scott TB, Allen GC, Heard PJ, Randell MG (2005) Reduction of U(VI) to U(IV) on the surface of magnetite. Geochim Cosmochim Acta 69:5639–5646
Shaheen SM, Frohne T, White JR, DeLaune RD, Rinklebe J (2017) Redox-induced mobilization of copper, selenium, and zinc in deltaic soils originating from Mississippi (USA) and Nile (Egypt) River Deltas: a better understanding of biogeochemical processes for safe environmental management. J Environ Manag 186:131–140
Sharmasarkar S, Vance GF (1995) Fractional partitioning for assessing solid-phase speciation and geochemical transformations of soil selenium. Soil Sci 160:43–55
Shen DZ, Fan JX, Zhou WZ, Gao BY, Yue QY, Kang Q (2009) Adsorption kinetics and isotherm of anionic dyes onto organo-bentonite from single and multisolute systems. J Hazard Mater 172(1):99–107
Shenasa M, Sainkar S, Lichtman D (1986) XPS study of some selected selenium compounds. J Electron Spectrosc Relat Phenom 40:329–337
Struyk Z, Sposito G (2001) Redox properties of standard humic acids. Geoderma 102:329–346
Tan JA, Zhu W, Wang W, Li R, Hou S, Wang D, Yang L (2002) Selenium in soil and endemic diseases in China. Sci Total Environ 284:227–235
Wan XM, Tandy S, Hockmann K, Schulin R (2013) Changes in Sb speciation with waterlogging of shooting range soils and impacts on plant uptake. Environ Pollut 172:53–60
Wang SS, Liang DL, Wang D, Wei W, Fu DD, Lin ZQ (2012) Selenium fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci Total Environ 427:159–164
Winkel L, Vriens B, Jones G, Schneider L, Pilon-Smits E, Bañuelos G (2015) Selenium cycling across soil-plant-atmosphere interfaces: a critical review. Nutrients 7:4199–41239
Xu W, Wang H, Liu R, Zhao X, Qu J (2011) Arsenic release from arsenic-bearing Fe–Mn binary oxide: effects of Eh condition. Chemosphere 83:1020–1027
Yamada H, Kase Y, Usuki M, Kajiyama S, Yonebayashi K (1999) Selective determination and formation of elemental selenium in soils. Soil Sci Plant Nutr 45:403–408
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci 254:2441–2449
Yang Z, Du M, Jiang J (2016) Reducing capacities and redox potentials of humic substances extracted from sewage sludge. Chemosphere 144:902–908
Zhang YQ, Moore JN (1996) Selenium fractionation and speciation in a wetland system. Environ Sci Technol 30:2613–2619
Zhang P, Sparks DL (1990) Kinetics of selenate and selenite adsorption/desorption at the goethite/water interface. Environ Sci Technol 24:1848–1856
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 41401255, 51508057), the Natural Science Foundation of Chongqing of China (No. cstc2015jcyjA20018), China Postdoctoral Science Foundation (2016M602633), Chongqing Postdoctoral Science Foundation Special Funded Project (Xm2017048), and Innovation Project of University Students’ Research in Chongqing (201710618024).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Kitae Baek
Electronic supplementary material
ESM 1
(DOC 37 kb)
Rights and permissions
About this article
Cite this article
Fan, J., Zeng, Y. & Sun, J. The transformation and migration of selenium in soil under different Eh conditions. J Soils Sediments 18, 2935–2947 (2018). https://doi.org/10.1007/s11368-018-1980-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-1980-9