Abstract
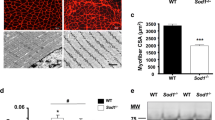
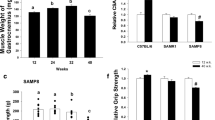
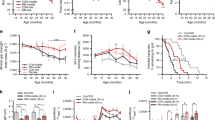
Mice lacking the superoxide anion scavenger CuZn superoxide dismutase (Sod1−/− mice) develop a number of age-related phenotypes, including an early progression of muscle atrophy and weakness (sarcopenia) associated with loss of innervation. The purpose of this study was to delineate the early development of sarcopenia in the Sod1−/− mice and to measure changes in the muscle transcriptome, proteome, and eicosanoid profile at the stage when sarcopenia is markedly induced in this model (7–9 months of age). We found a strong correlation between muscle atrophy and mitochondrial state 1 hydroperoxide production, which was 40% higher in isolated mitochondria from Sod1−/− mouse gastrocnemius muscle by 2 months of age. The primary pathways showing altered gene expression in Sod1−/− mice identified by RNA-seq transcriptomic analysis are protein ubiquitination, synaptic long-term potentiation, calcium signaling, phospholipase C signaling, AMPK, and TWEAK signaling. Targeted proteomics shows elevated expression of mitochondrial proteins, fatty acid metabolism enzymes, tricarboxylic acid (TCA) cycle enzymes, and antioxidants, while enzymes involved in carbohydrate metabolism are downregulated in Sod1−/− mice. LC-MS analysis of lipids in gastrocnemius muscle detected 78 eicosanoids, of which 31 are significantly elevated in muscle from Sod1−/− mice. These data suggest that mitochondrial hydroperoxide generation is elevated prior to muscle atrophy and may be a potential driving factor of changes in the transcriptome, proteome, and eicosanoid profile of the Sod1−/− mice. Together, these analyses revealed important molecular events that occur during muscle atrophy, which will pave the way for future studies using new approaches to treat sarcopenia.







Similar content being viewed by others
References
Ahn B, Pharaoh G, Premkumar P, Huseman K, Ranjit R, Kinter M, et al. Nrf2 deficiency exacerbates age-related contractile dysfunction and loss of skeletal muscle mass. Redox Biol. 2018;17:47–58.
Altun M, Besche HC, Overkleeft HS, Piccirillo R, Edelmann MJ, Kessler BM, et al. Muscle wasting in aged, sarcopenic rats is associated with enhanced activity of the ubiquitin proteasome pathway. J Biol Chem. 2010;285:39597–608.
Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–81.
Appell HJ, Duarte JA, Soares JM. Supplementation of vitamin E may attenuate skeletal muscle immobilization atrophy. Int J Sports Med. 1997;18:157–60.
Arnardottir HH, Dalli J, Norling LV, Colas RA, Perretti M, Serhan CN. Resolvin D3 is dysregulated in arthritis and reduces arthritic inflammation. J Immunol. 2016;197:2362–8.
Arrigoni E, Averet N, Cohadon F. Effects of CDP-choline on phospholipase A2 and cholinephosphotransferase activities following a cryogenic brain injury in the rabbit. Biochem Pharmacol. 1987;36:3697–700.
Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. 2005;41:173–86.
Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. 2017;96:10–5.
Bhattacharya A, Muller FL, Liu Y, Sabia M, Liang H, Song W, et al. Denervation induces cytosolic phospholipase A2-mediated fatty acid hydroperoxide generation by muscle mitochondria. J Biol Chem. 2009;284:46–55.
Bhattacharya A, Hamilton R, Jernigan A, Zhang Y, Sabia M, Rahman MM, et al. Genetic ablation of 12/15-lipoxygenase but not 5-lipoxygenase protects against denervation-induced muscle atrophy. Free Radic Biol Med. 2014;67:30–40.
Bilodeau PA, Coyne ES, Wing SS. The ubiquitin proteasome system in atrophying skeletal muscle: roles and regulation. Am J Phys Cell Physiol. 2016;311:C392–403.
Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Phys Cell Physiol. 2004;287:C475–83.
Cade WT. The manifold role of the mitochondria in skeletal muscle insulin resistance. Curr Opin Clin Nutr metab Care. 2018;21:267–72.
Calder PC. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–19S.
Chen C, Ju R, Zhu L, Li J, Chen W, Zhang DC, et al. Carboxyamidotriazole alleviates muscle atrophy in tumor-bearing mice by inhibiting NF-kappaB and activating SIRT1. Naunyn Schmiedeberg's Arch Pharmacol. 2017;390:423–33.
Chucair-Elliott AJ, Ocanas SR, Stanford DR, Hadad N, Wronowski B, Otalora L, et al. Tamoxifen induction of Cre recombinase does not cause long-lasting or sexually divergent responses in the CNS epigenome or transcriptome: implications for the design of aging studies. Geroscience. 2019;41:691–708.
Comim CM, Hoepers A, Ventura L, Freiberger V, Dominguini D, Mina F, et al. Activity of Krebs cycle enzymes in mdx mice. Muscle Nerve. 2016;53:91–5.
Cruz-Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13:1–7.
Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012;2012:646354-67.
Custodero C, Mankowski RT, Lee SA, Chen Z, Wu S, Manini TM, et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: a systematic review and meta-analysis. Ageing Res Rev. 2018;46:42–59.
Dalle C, Ostermann AI, Konrad T, Coudy-Gandilhon C, Decourt A, Barthelemy JC, et al. Muscle loss associated changes of oxylipin signatures during biological aging: an exploratory study from the PROOF cohort. J Gerontol A Biol Sci Med Sci. 2019;74:608–15.
Debold EP. Potential molecular mechanisms underlying muscle fatigue mediated by reactive oxygen and nitrogen species. Front Physiol. 2015;6:239.
Dogra C, Changotra H, Mohan S, Kumar A. Tumor necrosis factor-like weak inducer of apoptosis inhibits skeletal myogenesis through sustained activation of nuclear factor-kappaB and degradation of MyoD protein. J Biol Chem. 2006;281:10327–36.
Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–23.
Ebert SM, Dyle MC, Kunkel SD, Bullard SA, Bongers KS, Fox DK, et al. Stress-induced skeletal muscle Gadd45a expression reprograms myonuclei and causes muscle atrophy. J Biol Chem. 2012;287:27290–301.
Egawa T, Goto A, Ohno Y, Yokoyama S, Ikuta A, Suzuki M, et al. Involvement of AMPK in regulating slow-twitch muscle atrophy during hindlimb unloading in mice. Am J Physiol Endocrinol Metab. 2015;309:E651–62.
Gillon A, Nielsen K, Steel C, Cornwall J, Sheard P. Exercise attenuates age-associated changes in motoneuron number, nucleocytoplasmic transport proteins and neuromuscular health. Geroscience. 2018;40:177–92.
Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43:12–21.
Hashimoto M, Inoue T, Katakura M, Hossain S, Mamun AA, Matsuzaki K, et al. Differential effects of docoosahexaenoic and arachidonic acid on fatty acid composition and myosin heavy chain-related genes of slow- and fast-twitch skeletal muscle tissues. Mol Cell Biochem. 2016;415:169–81.
Hoxha M. Duchenne muscular dystrophy: focus on arachidonic acid metabolites. Biomed Pharmacother. 2019;110:796–802.
Huang TT, Yasunami M, Carlson EJ, Gillespie AM, Reaume AG, Hoffman EK, et al. Superoxide-mediated cytotoxicity in superoxide dismutase-deficient fetal fibroblasts. Arch Biochem Biophys. 1997;344:424–32.
Ivannikov MV, Van Remmen H. Sod1 gene ablation in adult mice leads to physiological changes at the neuromuscular junction similar to changes that occur in old wild-type mice. Free Radic Biol Med. 2015;84:254–62.
Jackson MJ. Lack of CuZnSOD activity: a pointer to the mechanisms underlying age-related loss of muscle function, a commentary on “absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy”. Free Radic Biol Med. 2006;40:1900–2.
Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24:1376–90.
Jang YC, Liu Y, Hayworth CR, Bhattacharya A, Lustgarten MS, Muller FL, et al. Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell. 2012;11:770–82.
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–5.
Kinter CS, Lundie JM, Patel H, Rindler PM, Szweda LI, Kinter M. A quantitative proteomic profile of the Nrf2-mediated antioxidant response of macrophages to oxidized LDL determined by multiplexed selected reaction monitoring. PLoS One. 2012;7:e50016.
Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56.
Kucukhuseyin O, Khalid S, Sabitaliyevich UY, Kucukhuseyin C. The role of PLC-IP3 cascade on 4-aminopyridine (4-AP) contracture in electrically-driven rat atrial and diaphragmatic strips: new evidence by neomycin and heparin. Cell Mol Biol (Noisy-le-Grand, France). 2018;64:26–32.
Kurahashi T, Hamashima S, Shirato T, Lee J, Homma T, Kang ES, et al. An SOD1 deficiency enhances lipid droplet accumulation in the fasted mouse liver by aborting lipophagy. Biochem Biophys Res Commun. 2015;467:866–71.
Kuwata H, Nakatani E, Shimbara-Matsubayashi S, Ishikawa F, Shibanuma M, Sasaki Y, et al. Long-chain acyl-CoA synthetase 4 participates in the formation of highly unsaturated fatty acid-containing phospholipids in murine macrophages. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:1606–18.
Lagerwaard B, Nieuwenhuizen AG, de Boer VCJ, Keijer J. In vivo assessment of mitochondrial capacity using NIRS in locomotor muscles of young and elderly males with similar physical activity levels. Geroscience. 2020;42:299–310.
Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol. 2011;589:2119–27.
Larkin LM, Davis CS, Sims-Robinson C, Kostrominova TY, Van Remmen H, Richardson A, et al. Skeletal muscle weakness due to deficiency of CuZn-superoxide dismutase is associated with loss of functional innervation. Am J Phys Regul Integr Comp Phys. 2011;301:R1400–7.
Liu J, Peng Y, Wang X, Fan Y, Qin C, Shi L, et al. Mitochondrial dysfunction launches dexamethasone-induced skeletal muscle atrophy via AMPK/FOXO3 signaling. Mol Pharm. 2016a;13:73–84.
Liu SZ, Jemiolo B, Lavin KM, Lester BE, Trappe SW, Trappe TA. Prostaglandin E2/cyclooxygenase pathway in human skeletal muscle: influence of muscle fiber type and age. J Appl Physiol (1985). 2016b;120:546–51.
Loehr JA, Stinnett GR, Hernandez-Rivera M, Roten WT, Wilson LJ, Pautler RG, et al. Eliminating Nox2 reactive oxygen species production protects dystrophic skeletal muscle from pathological calcium influx assessed in vivo by manganese-enhanced magnetic resonance imaging. J Physiol. 2016;594:6395–405.
Logan S, Royce GH, Owen D, Farley J, Ranjo-Bishop M, Sonntag WE, et al. Accelerated decline in cognition in a mouse model of increased oxidative stress. Geroscience. 2019;41:591–607.
Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, et al. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev. 2006;127:298–306.
McClung JM, Judge AR, Talbert EE, Powers SK. Calpain-1 is required for hydrogen peroxide-induced myotube atrophy. Am J Phys Cell Physiol. 2009;296:C363–71.
McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999;20:1–8.
McFadden SL, Ding D, Salvi R. Anatomical, metabolic and genetic aspects of age-related hearing loss in mice. Audiology. 2001;40:313–21.
Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43:W566–70.
Mittal A, Bhatnagar S, Kumar A, Lach-Trifilieff E, Wauters S, Li H, et al. The TWEAK-Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J Cell Biol. 2010;188:833–49.
Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004.
Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, et al. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Phys Regul Integr Comp Phys. 2007;293:R1159–68.
Nakao R, Shimba S, Oishi K. Ketogenic diet induces expression of the muscle circadian gene Slc25a25 via neural pathway that might be involved in muscle thermogenesis. Sci Rep. 2017;7:2885.
Nelson ME, Parker BL, Burchfield JG, Hoffman NJ, Needham EJ, Cooke KC, et al. Phosphoproteomics reveals conserved exercise-stimulated signaling and AMPK regulation of store-operated calcium entry. EMBO J. 2019;38:e102578.
Nilsson MI, Greene NP, Dobson JP, Wiggs MP, Gasier HG, Macias BR, et al. Insulin resistance syndrome blunts the mitochondrial anabolic response following resistance exercise. Am J Physiol Endocrinol Metab. 2010;299:E466–74.
Novak ML, Billich W, Smith SM, Sukhija KB, McLoughlin TJ, Hornberger TA, et al. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Phys Regul Integr Comp Phys. 2009;296:R1132–9.
Olofsson EM, Marklund SL, Behndig A. Glucose-induced cataract in CuZn-SOD null lenses: an effect of nitric oxide? Free Radic Biol Med. 2007;42:1098–105.
Pant M, Bal NC, Periasamy M. Sarcolipin: a key thermogenic and metabolic regulator in skeletal muscle. Trends Endocrinol Metab. 2016;27:881–92.
Pharaoh G, Sataranatarajan K, Street K, Hill S, Gregston J, Ahn B, et al. Metabolic and stress response changes precede disease onset in the spinal cord of mutant SOD1 ALS mice. Front Neurosci. 2019;13:487.
Piekarz KM, Bhaskaran S, Sataranatarajan K, Street K, Premkumar P, Saunders D, et al. Molecular changes associated with spinal cord aging. Geroscience. 2020. doi: https://doi.org/10.1007/s11357-020-00172-6.
Pompeia C, Freitas JJ, Kim JS, Zyngier SB, Curi R. Arachidonic acid cytotoxicity in leukocytes: implications of oxidative stress and eicosanoid synthesis. Biol Cell. 2002;94:251–65.
Powers SK, Morton AB, Ahn B, Smuder AJ. Redox control of skeletal muscle atrophy. Free Radic Biol Med. 2016;98:208–17.
Qaisar R, Bhaskaran S, Premkumar P, Ranjit R, Natarajan KS, Ahn B, et al. Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J Cachexia Sarcopenia Muscle. 2018;9:1003–17.
Qaisar R, Bhaskaran S, Ranjit R, Sataranatarajan K, Premkumar P, Huseman K, et al. Restoration of SERCA ATPase prevents oxidative stress-related muscle atrophy and weakness. Redox Biol. 2019;20:68–74.
Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–305.
Quehenberger O, Dahlberg-Wright S, Jiang J, Armando AM, Dennis EA. Quantitative determination of esterified eicosanoids and related oxygenated metabolites after base hydrolysis. J Lipid Res. 2018;59:2436–45.
Rodriguez J, Caille O, Ferreira D, Francaux M. Pomegranate extract prevents skeletal muscle of mice against wasting induced by acute TNF-alpha injection. Mol Nutr Food Res. 2017;61: 1600169-81.
Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55:M716–24.
Rudolf R, Khan MM, Labeit S, Deschenes MR. Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci. 2014;6:99.
Sakellariou GK, McDonagh B, Porter H, Giakoumaki II, Earl KE, Nye GA, et al. Comparison of whole body SOD1 knockout with muscle-specific SOD1 knockout mice reveals a role for nerve redox signaling in regulation of degenerative pathways in skeletal muscle. Antioxid Redox Signal. 2018;28:275–95.
Sakiyama H, Fujiwara N, Yoneoka Y, Yoshihara D, Eguchi H, Suzuki K. Cu,Zn-SOD deficiency induces the accumulation of hepatic collagen. Free Radic Res. 2016;50:666–77.
Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013;45:2121–9.
Sandri M, El Meslemani AH, Sandri C, Schjerling P, Vissing K, Andersen JL, et al. Caspase 3 expression correlates with skeletal muscle apoptosis in Duchenne and facioscapulo human muscular dystrophy. A potential target for pharmacological treatment? J Neuropathol Exp Neurol. 2001;60:302–12.
Sataranatarajan K, Qaisar R, Davis C, Sakellariou GK, Vasilaki A, Zhang Y, et al. Neuron specific reduction in CuZnSOD is not sufficient to initiate a full sarcopenia phenotype. Redox Biol. 2015;5:140–8.
Sauc S, Frieden M. Neurological and motor disorders: TRPC in the skeletal muscle. Adv Exp Med Biol. 2017;993:557–75.
Shi Y, Ivannikov MV, Walsh ME, Liu Y, Zhang Y, Jaramillo CA, et al. The lack of CuZnSOD leads to impaired neurotransmitter release, neuromuscular junction destabilization and reduced muscle strength in mice. PLoS One. 2014;9:e100834.
Siparsky PN, Kirkendall DT, Garrett WE Jr. Muscle changes in aging: understanding sarcopenia. Sports Health. 2014;6:36–40.
Sivakumar AS, Hwang I. Effects of Sunphenon and Polyphenon 60 on proteolytic pathways, inflammatory cytokines and myogenic markers in H2O2-treated C2C12 cells. J Biosci. 2015;40:53–9.
Sopariwala DH, Pant M, Shaikh SA, Goonasekera SA, Molkentin JD, Weisleder N, et al. Sarcolipin overexpression improves muscle energetics and reduces fatigue. J Appl Physiol (1985). 2015;118:1050–8.
Standley RA, Liu SZ, Jemiolo B, Trappe SW, Trappe TA. Prostaglandin E2 induces transcription of skeletal muscle mass regulators interleukin-6 and muscle RING finger-1 in humans. Prostaglandins Leukot Essent Fat Acids. 2013;88:361–4.
Sun K, Ma R, Cui X, Campos B, Webster R, Brockman D, et al. Glucocorticoids induce cytosolic phospholipase A2 and prostaglandin H synthase type 2 but not microsomal prostaglandin E synthase (PGES) and cytosolic PGES expression in cultured primary human amnion cells. J Clin Endocrinol Metab. 2003;88:5564–71.
Thomson DM. The role of AMPK in the regulation of skeletal muscle size, hypertrophy, and regeneration. Int J Mol Sci. 2018;19: 3125-45
Tithof PK, Roberts MP, Guan W, Elgayyar M, Godkin JD. Distinct phospholipase A2 enzymes regulate prostaglandin E2 and F2alpha production by bovine endometrial epithelial cells. Reprod Biol Endocrinol. 2007;5:16.
Tupling AR, Bombardier E, Gupta SC, Hussain D, Vigna C, Bloemberg D, et al. Enhanced Ca2+ transport and muscle relaxation in skeletal muscle from sarcolipin-null mice. Am J Phys Cell Physiol. 2011;301:C841–9.
Vaughan VC, Sullivan-Gunn M, Hinch E, Martin P, Lewandowski PA. Eicosapentaenoic acid and oxypurinol in the treatment of muscle wasting in a mouse model of cancer cachexia. PLoS One. 2012;7:e45900.
Vella L, Markworth JF, Farnfield MM, Maddipati KR, Russell AP, Cameron-Smith D. Intramuscular inflammatory and resolving lipid profile responses to an acute bout of resistance exercise in men. Phys Rep. 2019;7:e14108.
Wang Y, Armando AM, Quehenberger O, Yan C, Dennis EA. Comprehensive ultra-performance liquid chromatographic separation and mass spectrometric analysis of eicosanoid metabolites in human samples. J Chromatogr A. 2014;1359:60–9.
Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol (1985). 2010;108:1376–82.
Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–5.
Yakabe M. Aging-related frailty and sarcopenia. The relationship between "inflammaging" and frailty/sarcopenia. Clin Calcium. 2018;28:1215–9.
Acknowledgments
The authors thank the Multiplex Protein Quantification Cores at Oklahoma Nathan Shock Center, Oklahoma, Clinical genomic center at Oklahoma Medical Research Foundation, Oklahoma and Lipidomics core, UCSD, La Jolla, CA, for sharing their expertise.
Funding
This work was supported by the National Institute of Health, National Institute of Aging (AG050676, AG051442) and Veterans Affairs (I01BX002595). Dr. Van Remmen is the recipient of a Senior Research Career Scientist award (1IK6BX005234) from the Department of Veterans Affairs.
Author information
Authors and Affiliations
Contributions
HVR designed the study. KS, GP, CG, RR, KSt, and CK performed experiments and collected data. KS, GP, KP, JW, MK, WF, AR, and HVR analyzed data. KS, GP, JB, and HVR wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Ethics approval
The current research was conducted in accordance with the recommendations of USDA guidelines by the Institutional Animal Care and Use Committee at Oklahoma Medical Research Foundation.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data available upon request.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PPTX 218 kb)
About this article
Cite this article
Sataranatarajan, K., Pharaoh, G., Brown, J.L. et al. Molecular changes in transcription and metabolic pathways underlying muscle atrophy in the CuZnSOD null mouse model of sarcopenia. GeroScience 42, 1101–1118 (2020). https://doi.org/10.1007/s11357-020-00189-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00189-x