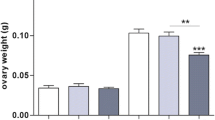
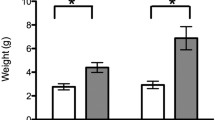
Abstract
Maternal protein restriction (MPR) in pregnancy causes life course organ dysfunction, but few studies link the developmental origins of disease hypothesis to early aging. Suboptimal developmental nutrition increases oxidative stress (OS) and male infertility, damaging sperm function. We hypothesized that MPR in pregnancy accelerates age-related changes in testicular and sperm function related to both maternal diet and increased testicular OS in rat offspring. We studied male rats whose pregnant mothers ate either control (C, 20 % casein) or restricted (R, 10 % casein) isocaloric diet. After birth, mothers and offspring ate C diet. Testes were retrieved at 19 days gestation and across the life course (postnatal day (PND) 21, 36, 110, and 850) to measure OS markers, antioxidant enzymes, serum FSH, LH, and testosterone, and PND 110 sperm OS and quality. Fertility rate was evaluated at PND 110, 450, and 850. Offspring showed age- and MPR-related changes in testosterone, testicular OS markers and antioxidant enzymes and fertility, and maternal diet-related OS and sperm antioxidant enzyme changes. Developmental programming is considered a key factor in predisposing to chronic disease. Our data show that programming also plays an important role in aging trajectory. This interaction is a little studied area in aging biology that merits more investigation.






Similar content being viewed by others
Abbreviations
- C:
-
Control
- dG:
-
Days of gestation DCF 2’,7’-dichlorofluorescein
- FSH:
-
Follicle-stimulating hormone
- GPx:
-
Glutathione peroxidase
- LH:
-
Luteinizing hormone
- MDA:
-
Malonaldialdehyde
- OS:
-
Oxidative stress
- PND:
-
Postnatal days
- RIA:
-
Radioimmunoassay
- ROS:
-
Reactive oxygen species
- R:
-
Restricted
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thiobarbituric acid-reactive substances assay
References
Agarwal A, Saleh RA, Bedaiwy MA (2003) Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 79:829–843
Aggerholm AS, Thulstrup AM, Toft G, Ramlau-Hansen CH, Bonde JP (2008) Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril 90:619–626. doi:10.1016/j.fertnstert.2007.07.1292
Aitken RJ, Roman SD (2008) Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev 1:15–24
Allen JA, Diemer T, Janus P, Hales KH, Hales DB (2004) Bacterial endotoxin lipopolysaccharide and reactive oxygen species inhibit Leydig cell steroidogenesis via perturbation of mitochondria. Endocrine 25:265–275
Barker DJ, Eriksson JG, Forsen T, Osmond C (2002) Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31:1235–1239
Bernal AB, Vickers MH, Hampton MB, Poynton RA, Sloboda DM (2010) Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS One 5:e15558. doi:10.1371/journal.pone.0015558
Bjorndahl L, Soderlund I, Kvist U (2003) Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum Reprod 18:813–816
Bonavera JJ et al (1997) In the male brown-Norway (BN) male rat, reproductive aging is associated with decreased LH-pulse amplitude and area. J Androl 18:359–365
Cabler S, Agarwal A, Flint M, du Plessis SS (2010) Obesity: modern man’s fertility nemesis. Asian J Androl 12:480–489. doi:10.1038/aja.2010.38
Davies MJ, Norman RJ (2002) Programming and reproductive functioning. Trends Endocrinol Metab 13:386–392
Diemer T, Allen JA, Hales KH, Hales DB (2003) Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology 144:2882–2891. doi:10.1210/en.2002-0090
Franco MC, Akamine EH, Reboucas N, Carvalho MH, Tostes RC, Nigro D, Fortes ZB (2007) Long-term effects of intrauterine malnutrition on vascular function in female offspring: implications of oxidative stress. Life Sci 80:709–715. doi:10.1016/j.lfs.2006.10.028
Free MJ, Schluntz GA, Jaffe RA (1976) Respiratory gas tensions in tissues and fluids of the male rat reproductive tract. Biol Reprod 14:481–488
Galjaard S, Devlieger R, Van Assche FA (2013) Fetal growth and developmental programming. J Perinat Med 41:101–105. doi:10.1515/jpm-2012-0020
Gluckman PD, Hanson MA, Pinal C (2005) The developmental origins of adult disease. Matern Child Nutr 1:130–141. doi:10.1111/j.1740-8709.2005.00020.x
Gluckman PD, Hanson MA, Beedle AS (2007) Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol 19:1–19. doi:10.1002/ajhb.20590
Gruenewald DA, Naai MA, Hess DL, Matsumoto AM (1994) The brown Norway rat as a model of male reproductive aging: evidence for both primary and secondary testicular failure. J Gerontol 49:B42–50
Guzman C, Cabrera R, Cardenas M, Larrea F, Nathanielsz PW, Zambrano E (2006) Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol 572:97–108
Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW (2008) Male obesity and alteration in sperm parameters. Fertil Steril 90:2222–2225. doi:10.1016/j.fertnstert.2007.10.011
Hardy MP, Schlegel PN (2004) Testosterone production in the aging male: where does the slowdown occur? Endocrinology 145:4439–4440. doi:10.1210/en.2004-0888
Heindel JJ (2008) Animal models for probing the developmental basis of disease and dysfunction paradigm. Basic Clin Pharmacol Toxicol 102:76–81. doi:10.1111/j.1742-7843.2007.00184.x
Hokao R, Saito TR, Wakafuji Y, Takahashi KW, Imamichi T (1993) The change with age of the copulatory behavior of the male rats aged 67 and 104 weeks. Jikken Dobutsu 42:75–82
Ingelfinger JR, Nuyt AM (2012) Impact of fetal programming, birth weight, and infant feeding on later hypertension. J Clin Hypertens (Greenwich) 14:365–371. doi:10.1111/j.1751-7176.2012.00660.x
Ivanova DG, Yankova TM (2013) The free radical theory of aging in search of a strategy for increasing life span. Folia Med 55:33–41
Landry D, Cloutier F, Martin LJ (2013) Implications of leptin in neuroendocrine regulation of male reproduction. Reprod Biol 13:1–14. doi:10.1016/j.repbio.2012.12.001
Luo ZC et al (2006) Tracing the origins of “fetal origins” of adult diseases: programming by oxidative stress? Med Hypotheses 66:38–44. doi:10.1016/j.mehy.2005.08.020
Luo ZC, Xiao L, Nuyt AM (2010) Mechanisms of developmental programming of the metabolic syndrome and related disorders. World J Diabetes 1:89–98. doi:10.4239/wjd.v1.i3.89
Martini AC, Molina RI, Ruiz RD, de Fiol Cuneo M (2012) Obesity and male fertility. Rev Fac Cien Med Univ Nac Cordoba 69:102–110
McMullen S, Mostyn A (2009) Animal models for the study of the developmental origins of health and disease. Proc Nutr Soc 68:306–320. doi:10.1017/S0029665109001396
Meeker JD, Godfrey-Bailey L, Hauser R (2007) Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl 28:397–406
Mutsaers HA, Tofighi R (2012) Dexamethasone enhances oxidative stress-induced cell death in murine neural stem cells. Neurotox Res 22:127–137. doi:10.1007/s12640-012-9308-9
Nathanielsz PW (2006) Animal models that elucidate basic principles of the developmental origins of adult diseases. ILAR J 47:73–82
World Health Organization (2010) WHO laboratory manual for the examination and processing of human semen. Fifth Edition edn. WHO Press, Switzerland
Ozanne SE, Hales CN (2004) Lifespan: catch-up growth and obesity in male mice. Nature 427:411–412. doi:10.1038/427411b
Palmer NO, Bakos HW, Fullston T, Lane M (2012) Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2:253–263. doi:10.4161/spmg.21362
Portha B, Chavey A, Movassat J (2011) Early-life origins of type 2 diabetes: fetal programming of the beta-cell mass. Exp Diabetes Res 2011:105076. doi:10.1155/2011/105076
Rae MT, Kyle CE, Miller DW, Hammond AJ, Brooks AN, Rhind SM (2002a) The effects of undernutrition, in utero, on reproductive function in adult male and female sheep. Anim Reprod Sci 72:63–71
Rae MT, Rhind SM, Kyle CE, Miller DW, Brooks AN (2002b) Maternal undernutrition alters triiodothyronine concentrations and pituitary response to GnRH in fetal sheep. J Endocrinol 173:449–455
Rodríguez-González GL, Vigueras-Villaseñor RM, Millán S, Moran N, Trejo R, Nathanielsz PW, Larrea F, Zambrano E (2012) Maternal protein restriction in pregnancy and/or lactation affects seminiferous tubule organization in male rat offspring. J Dev Orig Health Dis 3:321–326. doi:10.1017/S2040174412000360
Sarr O, Yang K, Regnault TR (2012) In utero programming of later adiposity: the role of fetal growth restriction. J Pregnancy 2012:134758. doi:10.1155/2012/134758
Sikka SC (1996) Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front Biosci : J Virtual Libr 1:e78–86
Tarry-Adkins JL, Ozanne SE (2011) Mechanisms of early life programming: current knowledge and future directions. Am J Clin Nutr 94:1765S–1771S. doi:10.3945/ajcn.110.000620
Tarry-Adkins JL, Chen JH, Jones RH, Smith NH, Ozanne SE (2010) Poor maternal nutrition leads to alterations in oxidative stress, antioxidant defense capacity, and markers of fibrosis in rat islets: potential underlying mechanisms for development of the diabetic phenotype in later life. FASEB J 24:2762–2771. doi:10.1096/fj.10-156075
Toledo FC, Perobelli JE, Pedrosa FP, Anselmo-Franci JA, Kempinas WD (2011) In utero protein restriction causes growth delay and alters sperm parameters in adult male rats. Reprod Biol Endocrinol 9:94
Vazquez-Memije ME, Capin R, Tolosa A, El-Hafidi M (2008) Analysis of age-associated changes in mitochondrial free radical generation by rat testis. Mol Cell Biochem 307:23–30. doi:10.1007/s11010-007-9580-9
Vega CC, Reyes-Castro LA, Bautista CJ, Larrea F, Nathanielsz PW, Zambrano E (2013) Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism Int J Obes (Lond) doi:10.1038/ijo.2013.150
Zambrano E, Nathanielsz PW (2013) Mechanisms by which maternal obesity programs offspring for obesity: evidence from animal studies. Nutr Rev 71(Suppl 1):S42–54. doi:10.1111/nure.12068
Zambrano E et al (2005a) Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol 566:225–236
Zambrano E et al (2005b) A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol 563:275–284
Zambrano E et al (2006) A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol 571:221–230
Zambrano E, Guzmán C, Rodríguez-González G, Durand-Carbajal M, Nathanielsz PW (2014a) Fetal programming of sexual development and reproductive function. Mol Cell Endocrinol 382:538–549
Zambrano E TN, Long N. M., Guo C, Sun K, Cox L. A., Ford S. P., Nathanielsz P. W. and Li C (2014b) Increased Central and Peripheral Glucocorticoid Synthesis Act As an Orchestrator of Developmental Programming. In: Zhang L PD, and Longo L.D, M.D. (ed) Stress and Developmental Programming of Health and Disease: Beyond Phenomenology. Nova Science Publishers, Inc, pp 463–486
Acknowledgments
Rodríguez-González GL and Reyes-Castro LA are graduate students from Doctorado en Ciencias Biomédicas, Facultad de Medicina, Universidad Nacional Autónoma de México, and Carlos Ibáñez is a graduate student from Doctorado en Ciencias Bioquímicas, Facultad de Química, Universidad Nacional Autónoma de México—recipients of Consejo Nacional de Ciencia y Tecnología (CONACyT) fellowship. This work was supported by CONACyT México 155166.
Conflict of interest
The authors have nothing to disclose and have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Rodríguez-González, G.L., Reyes-Castro, L.A., Vega, C.C. et al. Accelerated aging of reproductive capacity in male rat offspring of protein-restricted mothers is associated with increased testicular and sperm oxidative stress. AGE 36, 9721 (2014). https://doi.org/10.1007/s11357-014-9721-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-014-9721-5