Abstract
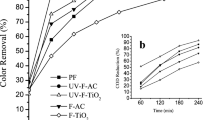
Fenton oxidation is an effective and valuable method for wastewater treatment. To inhibit environmental impacts and increase overall reaction efficiencies, it is important to develop advanced catalysts. This paper illustrates an experimental study on the elimination of RR180 dye from synthetic aqueous solutions with raw leonardite and different iron-loaded leonardite powders, Fe(0)-loaded leonardite, and Fe(II)-loaded leonardite. The effect of solution pH (2.0–6.0), catalyst amount (0.10–1.5 g/L), H2O2 concentration (10–50 µL/L), and dye concentration (10–30 ppm) was tested to achieve maximum color removal efficiency using the three catalysts. At pH = 2, color removal efficiencies were higher and more suitable. Initial experiments showed the advantage of using Fe(II)-loaded leonardite on using Fe(0)-loaded leonardite. Fe(II)-loaded leonardite catalyst was the most efficient in RR180 color removal compared to the other tested reagents. Color removal in function of solution pH did not decrease much when Fe(II)-loaded leonardite was used (100 to 96%) when pH was increased from 2.0 to 6.0. In the other hand, dye removal has been significantly affected in the case of using raw leonardite, Fe(0)-loaded leonardite (93 to 0%), and (100 to 13%) in the same pH range, respectively. At optimum experimental conditions, catalyst amount: 0.75 g/L for Fe(II) and Fe(0)-loaded leonardite and 1.5 g/L for raw leonardite; dye concentration: 10 ppm; solution pH: 2.0; H2O2 concentration: 50 µL/L; volume: 100 mL and reaction time: 60 min, RR180 dye removal efficiencies were 91%, 100%, and 100% by raw leonardite, Fe(0)-loaded leonardite and Fe(II)-loaded leonardite, respectively. The stability and reusability of the tested catalyst was investigated up to ten cycles. The experimental results revealed that both Fe(0)-loaded leonardite and Fe(II)-loaded leonardite can be used in Fenton reaction up to four cycles without decreasing their efficiency in RR180 color removal. The characterization of the catalysts was established using scanning electron microscope with energy dispersive X-ray spectroscopy (SEM–EDX). The synthesized catalyst can be used at large scale in any textile industry to effectively remove dyes resulting in high elimination rates at the optimal determined and studied conditions.








Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abukhadra MR, Adlii A, MohamedBakry B (2019) Green fabrication of bentonite/chitosan@cobalt oxide composite (BE/ CH@Co) of enhanced adsorption and advanced oxidation removal of Congo red dye and Cr (VI) from water. Int J Biol Macromol 126:402–413
Acisli O, Khataee A, Karaca S, Karimi A, Dogan E (2017) Combination of ultrasonic and Fenton processes in the presence of magnetite nanostructures prepared by high energy planetary ball mill. Ultrason Sonochem 34:754–762. https://doi.org/10.1016/j.ultsonch.2016.07.011
Albadarin AB, Mangwandi C (2015) Mechanisms of Alizarin Red S and Methylene blue biosorption onto olive stone by-product: isotherm study in single and binary systems. J Environ Manage 164:86–93. https://doi.org/10.1016/j.jenvman.2015.08.040
Arslan H, Saleh M, Bilici Z, Dizge N (2022a) Leonardite powder as an efficient adsorbent for cationic and anionic dyes. Water Environ Res 94(5):e10719. https://doi.org/10.1002/wer.10719
Arslan H, Eskikaya O, Bilici Z, Dizge N, Balakrishnan D (2022b) Comparison of Cr(VI) adsorption and photocatalytic reduction efficiency using leonardite powder. Chemosphere 300:134492. https://doi.org/10.1016/j.chemosphere.2022.134492
Babuponnusami A, Muthukumar K (2014) A review on Fenton and improvements to the Fenton process for wastewater treatment. J Environ Chem Eng 2(1):557–572. https://doi.org/10.1016/j.jece.2013.10.011
Bautista P, Mohedano AF, Gilarranz MA, Casas JA, Rodriguez JJ (2007) Application of Fenton oxidation to cosmetic wastewaters treatment. J Hazard Mater 143(1–2):128–134. https://doi.org/10.1016/j.jhazmat.2006.09.004
Baycan N, Can B (2019) Color removal from yeast production industry wastewater using photo-Fenton process. Pamukkale Univ J Eng Sci 25(3):292–296. https://doi.org/10.5505/pajes.2018.78872
Bilici Z, Bouchareb R, Sacak T, Yatmaz HC, Dizge N (2021) Recycling of TiO2-containing waste and utilization by photocatalytic degradation of a reactive dye solution. Water Sci Technol 83(5):1242–1249. https://doi.org/10.2166/wst.2020.606
Bouchareb R, Derbal K, Özay Y, Bilici Z, Dizge N (2020) Combined natural/chemical coagulation and membrane filtration for wood processing wastewater treatment. J Water Process Eng 37(July):101521. https://doi.org/10.1016/j.jwpe.2020.101521
Bouras HD, Yeddou AR, Bouras N, Hellel D, Holtz MD, Sabaou N, Chergui A, Nadjemi B (2017) Biosorption of Congo red dye by Aspergillus carbonarius M333 and Penicillium glabrum Pg1: kinetics, equilibrium and thermodynamic studies. J Taiwan Inst Chem Eng 80:915–923. https://doi.org/10.1016/j.jtice.2017.08.002
Çiner F, Gökkuş Ö (2012) Treatability of dye solutions containing disperse dyes by Fenton and Fenton-solar light oxidation processes. Clean Soil Air Water 41(1):80–85. https://doi.org/10.1002/clen.201000500
Demissie H, An G, Jiao R, Ritigala T, Lu S, Wang D (2021) Modification of high content nanocluster-based coagulation for rapid removal of dye from water and the mechanism. Sep Purif Technol 259(March):117845. https://doi.org/10.1016/j.seppur.2020.117845
Deniz F, Kepekci RA (2016) Dye biosorption onto pistachio by-product: a green environmental engineering approach. J Mol Liq 219:194–200. https://doi.org/10.1016/j.molliq.2016.03.018
El-Desoky HS, Ghoneim MM, El-Sheikh R, Zidan NM (2010) Oxidation of Levafix CA reactive azo-dyes in industrial wastewater of textile dyeing by electro-generated Fenton’s reagent. J Hazard Mater 175(1–3):858–865. https://doi.org/10.1016/j.jhazmat.2009.10.089
Es’haghzade Z, Pajootan E, Bahrami H, Arami M (2017) Facile synthesis of Fe3O4 nanoparticles via aqueous based electro chemical route for heterogeneous electro-Fenton removal of azo dyes. J Taiwan Inst Chem Eng 71:91–105. https://doi.org/10.1016/j.jtice.2016.11.015
Gökkuş Ö, Yıldız YŞ (2014) Investigation of the effect of process parameters on the coagulation- investigation of the effect of process parameters on the coagulation flocculation treatment of textile wastewater using the Taguchi experimental method. Fresenius Environ Bull 23(02):463–470
Gökku Ö, Ço F, Kocaoglu M, Yıldız YŞ (2013) Determination of optimum conditions for color and COD removal of Reactive Blue 19 by Fenton oxidation process. Desalin Water Treat:37–41. https://doi.org/10.1080/19443994.2013.812523
Gusain R, Kumar N, Ray SS (2020) Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord Chem Rev 405:213111. https://doi.org/10.1016/j.ccr.2019.213111
Hassani A, Karaca C, Karaca S, Khataee A, Açışlı Ö, Yılmaz B (2018) Enhanced removal of basic violet 10 by heterogeneous sono-Fenton process using magnetite nanoparticles. Ultrason Sonochem 42:390–402. https://doi.org/10.1016/j.ultsonch.2017.11.036
Ivanets A, Roshchina M, Srivastava V, Prozorovich V, Dontsova T, Nahirniak S, Pankov V, Hosseini-Bandegharaei A, Nguyen Tran H, Sillanpää M (2019) Effect of metal ions adsorption on the efficiency of methylene blue degradation onto MgFe 2 O 4 as Fenton-like catalysts. Colloids Surf A Physicochem Eng Asp 571:17–26. https://doi.org/10.1016/j.colsurfa.2019.03.071
Januário EFD, Vidovix TB, Bergamasco R, Vieira AMS (2021) Performance of a hybrid coagulation/flocculation process followed by modified microfiltration membranes for the removal of solophenyl blue dye. Chem Eng Process Process Intensif 168(March). https://doi.org/10.1016/j.cep.2021.108577
Karthikeyan S, Titus A, Gnanamani A, Mandal AB, Sekaran G (2011) Treatment of textile wastewater by homogeneous and heterogeneous Fenton oxidation processes. Desalination 281(1):438–445. https://doi.org/10.1016/j.desal.2011.08.019
Kim TH, Park C, Yang J, Kim S (2004) Comparison of disperse and reactive dye removals by chemical coagulation and Fenton oxidation. J Hazard Mater 112(1–2):95–103. https://doi.org/10.1016/j.jhazmat.2004.04.008
Kousha M, Tavakoli S, Daneshvar E, Vazirzadeh A, Bhatnagar A (2015) Central composite design optimization of Acid Blue 25 dye biosorption using shrimp shell biomass. J Mol Liq 207:266–273. https://doi.org/10.1016/j.molliq.2015.03.046
Martínez CM, Celis LB, Cervantes FJ (2013) Immobilized humic substances as redox mediator for the simultaneous removal of phenol and Reactive Red 2 in a UASB reactor. Appl Microbiol Biotechnol 97(22):9897–9905. https://doi.org/10.1007/s00253-013-5190-5
Moussavi M, Matavos-Aramyan S (2016) Chelate-modified fenton treatment of sulfidic spent caustic. Korean J Chem Eng 33(8):2384–2391. https://doi.org/10.1007/s11814-016-0080-z
Nidheesh PV, Gandhimathi R (2012) Trends in electro-Fenton process for water and wastewater treatment: an overview. Desalination 299:1–15. https://doi.org/10.1016/j.desal.2012.05.011
Pani N, Tejani V, Anantha-Singh TS, Kandya A (2020) Simultaneous removal of COD and ammoniacal nitrogen from dye intermediate manufacturing Industrial Wastewater using Fenton oxidation method. Appl Water Sci 10(2):1–7. https://doi.org/10.1007/s13201-020-1151-1
Pelosi BT, Lima LKS, Vieira MGA (2014) Removal of the synthetic dye remazol brilliant blue r from textile industry wastewaters by biosorption on the macrophyte Salvinia natans. Braz J Chem Eng 31(4):1035–1045. https://doi.org/10.1590/0104-6632.20140314s00002568
Raji M, Mirbagheri SA, Ye F, Dutta J (2021) Nano zero-valent iron on activated carbon cloth support as Fenton-like catalyst for efficient color and COD removal from melanoidin wastewater. Chemosphere 263:127945. https://doi.org/10.1016/j.chemosphere.2020.127945
Ramirez JH, Maldonado-Hodar FJ, Pérez-Cadenas AF, Moreno-castilla C, Costa CA, Madeira LM (2007) Azo-dye Orange II degradation by heterogeneous Fenton-like reaction using carbon-Fe catalysts. Appl Catal B 75:312–323. https://doi.org/10.1016/j.apcatb.2007.05.003
Sathishkumar P, Arulkumar M, Palvannan T (2012) Utilization of agro-industrial waste Jatropha curcas pods as an activated carbon for the adsorption of reactive dye Remazol Brilliant Blue R (RBBR). J Clean Prod 22(1):67–75. https://doi.org/10.1016/j.jclepro.2011.09.017
Sayjumpa J, Jomhataikool B, Faungnawakij K, Kuboon S, Kraithong W, Fuji M, Eiad-Ua A (2019) Porous carbon adsorbent from humin derived from thai leonardite for methylene blue dye adsorption. Curr Appl Sci Technol 19(1):1–8. https://doi.org/10.14456/cast.2019.1
Shen Z, Wang W, Jia J, Ye J, Feng X, Peng A (2001) Degradation of dye solution by an activated carbon fiber electrode electrolysis system. J Hazard Mater 84(1):107–116. https://doi.org/10.1016/S0304-3894(01)00201-1
Sievers M (2011) 4.13 - Advanced oxidation processes. In: Wilderer P (ed) Treatise on water science. Elsevier, pp 377–408. https://doi.org/10.1016/B978-0-444-53199-5.00093-2, https://www.sciencedirect.com/science/article/pii/B9780444531995000932
Singa PK, Isa MH, Ho YC, Lim JW (2018) Treatment of hazardous waste landfill leachate using Fenton oxidation process. E3S Web Conf 34:4–9. https://doi.org/10.1051/e3sconf/20183402034
Solé-Sardans M, Gamisans X, Dorado AD, Lao-Luque C (2016) Exploring arsenic adsorption at low concentration onto modified leonardite. Water Air Soil Pollut 227(4). https://doi.org/10.1007/s11270-016-2827-x
Terdputtakun A, Arqueropanyo O. anong, Sooksamiti P, Janhom S, Naksata W (2017) Adsorption isotherm models and error analysis for single and binary adsorption of Cd(II) and Zn(II) using leonardite as adsorbent. Environ Earth Sci 76(22):1–11. https://doi.org/10.1007/s12665-017-7110-y
Tony MA, Parker HL, Clark JH (2016) Treatment of laundrette wastewater using Starbon and Fenton’s reagent. J Environ Sci Health A Tox Hazard Subst Environ Eng 51(11):974–979. https://doi.org/10.1080/10934529.2016.1191817
Yang H, Shi B, Wang S (2018) Fe oxides loaded on carbon cloth by hydrothermal process as an effective and reusable heterogenous Fenton catalyst. Catalysts, 8(5). https://doi.org/10.3390/catal8050207
Zhang MF, Qin YH, Ma JY, Yang L, Wu ZK, Wang TL, Wang WG, Wang CW (2016) Depolymerization of microcrystalline cellulose by the combination of ultrasound and Fenton reagent. Ultrason Sonochem 31:404–408. https://doi.org/10.1016/j.ultsonch.2016.01.027
Zhong J, Yang B, Feng Y, Chen Y, Wang LG, You WD, Ying GG (2021) Enhanced photo-fenton removal efficiency with core-shell magnetic resin catalyst for textile dyeing wastewater treatment. Water (Switzerland) 13(7). https://doi.org/10.3390/w13070968
Author information
Authors and Affiliations
Contributions
ZB carried out the methodology. HA and ND found leonardite powder for Fenton oxidation and they had the main idea. RB and ND were a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arslan, H., Bouchareb, R., Arikan, E.B. et al. Iron-loaded leonardite powder for Fenton oxidation of Reactive Red 180 dye removal. Environ Sci Pollut Res 29, 77071–77080 (2022). https://doi.org/10.1007/s11356-022-21306-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21306-7