Abstract
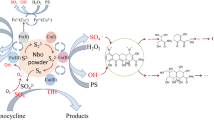
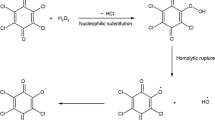
Both horseradish peroxidase (HRP) and ferrous ion (Fe2+) can degrade organic micropollutants (e.g., sulfamethazine) in the presence of hydrogen peroxide (H2O2), but they have their own disadvantages, such as low degradation efficiency and low pH condition, respectively. In order to overcome the above shortcomings, this study is to develop a HRP-Fenton-like system for sulfamethazine (SMR) efficient degradation by analyzing the optimal reaction conditions, degradation mechanisms, and effect of ions. Results show that HRP and Fe2+ achieve effective coupling by adding trace Fe2+ (≤ 10 μmol/mL) to the HRP system at pH = 5, and the degradation rate of SMR increased by 20.7–42% depending on Fe2+ concentration compared with single HRP treatment. Consumption of H2O2 and quenching of hydroxyl radicals confirmed that HRP dominated SMR removal in the HRP-Fenton-like system. HPLC-MS analysis shows that SMR was degraded by C-S bond breaking, N-S bond breaking, hydroxyl substitution, and rearrangement. Furthermore, Cl−, HCO3−, and NO3− exhibit an acceptable negative effect, while SO42− shows a positive effect on the degradation of SMR.







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al-Maqdi KA, Hisaindee S, Rauf MA, Ashraf SS (2018) Detoxification and degradation of sulfamethoxazole by soybean peroxidase and UV + H2O2 remediation approaches. Chem Eng J 352:450–458
Anjali R, Shanthakumar S (2019) Insights on the current status of occurrence and removal of antibiotics in wastewater by advanced oxidation processes. J Environ Manag 246:51–62
Berglund GI, Carlsson GH, Smith AT, Szöke H, Henriksen A, Hajdu J (2002) The catalytic pathway of horseradish peroxidase at high resolution. Nature 417:463–468
Bilal M, Ashraf SS, Barceló D, Iqbal HMN (2019) Biocatalytic degradation/redefining “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci Total Environ 691:1190–1211
Boreen AL, Arnold WA, Mcneill K (2004) Photochemical fate of sulfa drugs in the aquatic environment: sulfa drugs containing five-membered heterocyclic groups. Environ Sci Technol 38:3933–3940
Chaturvedi NK, Katoch SS (2020a) Evaluation and comparison of Fenton-like oxidation with Fenton’s oxidation for hazardous methoxyanilines in aqueous solution. J Ind Eng Chem 92:101–108
Chaturvedi NK, Katoch SS (2020b) Remedial technologies for aniline and aniline derivatives elimination from wastewater. J Health Pollut 10:200302
Colosi LM, Burlingame DJ, Huang Q, Webr JW Jr (2007) Peroxidase-mediated removal of a polychlorinated biphenyl using natural organic matter as the sole cosubstrate. Environ Ence Technol 41:891–896
Dao YH, De Laat J (2011) Hydroxyl radical involvement in the decomposition of hydrogen peroxide by ferrous and ferric-nitrilotriacetate complexes at neutral pH. Water Res 45:3309–3317
Du J, Xiao G, Xi Y, Zhu X, Su F, Kim SH (2020) Periodate activation with manganese oxides for sulfanilamide degradation. Water Res 169:115278
Fernandes M, Souza DH, Henriques RO, Alves MV, Skoronski E, Junior AF (2020) Obtaining soybean peroxidase from soybean hulls and its application for detoxification of 2,4-dichlorophenol contaminated water. J Environ Chem Eng 8:103786
Garcia-Galan MJ, Silvia Diaz-Cruz M, Barcelo D (2008) Identification and determination of metabolites and degradation products of sulfonamide antibiotics. Trac Trends in Analytical Chemistry
Guo J, Liu X, Zhang X, Wu J, Ge W (2019) Immobilized lignin peroxidase on Fe3O4@SiO2@polydopamine nanoparticles for degradation of organic pollutants. Int J Biol Macromol 138:433–440
Hou G, Zhang R, Hao X, Liu C (2017) An exploration of the effect and interaction mechanism of bisphenol A on waste sludge hydrolysis with multi-spectra, isothermal titration microcalorimetry and molecule docking. J Hazard Mater 333:32–41
Imlay JA, Chin SM, Linn S (1988) Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240:640–642
Jiang W, Yang J, Wang X, Han H, Yang Y, Tang J, Li Q (2017) Phenol degradation catalyzed by a peroxidase mimic constructed through the grafting of heme onto metal-organic frameworks. Bioresource Technology, S0960852417317261
Jing M, Han G, Wan J, Zhang S, Yang J, Zong W, Niu Q, Liu R (2020) Catalase and superoxide dismutase response and the underlying molecular mechanism for naphthalene. Sci Total Environ 736:139567
Jun LY, Yon LS, Mubarak NM, Bing CH, Pan S, Danquah MK, Abdullah EC, Khalid M (2019) An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J Environ Chem Eng 7:102961
Keshta BE, Gemeay AH, Khamis AA (2021) Impacts of horseradish peroxidase immobilization onto functionalized superparamagnetic iron oxide nanoparticles as a biocatalyst for dye degradation. Environ Sci Pollut Res
Kim J, Choe YJ, Kim SH, Jeong K (2019) Enhancing the decomposition of refractory contaminants on SO42--functionalized iron oxide to accommodate surface SO4- generated via radical transfer from OH. Appl Catal B Environ 252:62–76
Liang C, He B (2018) A titration method for determining individual oxidant concentration in the dual sodium persulfate and hydrogen peroxide oxidation system. Chemosphere 198:297–302
Magario I, García Einschlag FS, Rueda EH, Zygadlo J, Ferreira ML (2012) Mechanisms of radical generation in the removal of phenol derivatives and pigments using different Fe-based catalytic systems. J Mol Catal A Chem 352:1–20
Morsi R, Bilal M, Iqbal HMN, Ashraf SS (2020) Laccases and peroxidases: the smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci Total Environ 714:136572
Msagati TAM, Ngila JC (2002) Voltammetric detection of sulfonamides at a poly(3-methylthiophene) electrode. Talanta 58:605–610
Poulos TL (2014) Heme enzyme structure and function. Chem Rev 114:3919–3962
Puri S, Thakur I, Verma A, Barman S (2021) Degradation of pharmaceutical drug paracetamol via UV irradiation using Fe-TiO2 composite photocatalyst: statistical analysis and parametric optimization. Environ Sci Pollut Res 28:47327–47341
Sarmah AK, Meyer MT, Boxall ABA (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759
Sopaj F, Oturan N, Pinson J, Podvorica FI, Oturan MA (2020) Effect of cathode material on electro-Fenton process efficiency for electrocatalytic mineralization of the antibiotic sulfamethazine. Chem Eng J 384:123249
Takaku T, Mikata K, Nagahori H, Sogame Y (2014) Identification of metabolites of propyrisulfuron in rats. J Chromatogr B 955-956:64–71
Tentscher PR, Eustis SN, Mcneill K, Arey JS (2013) Aqueous oxidation of sulfonamide antibiotics: aromatic nucleophilic substitution of an aniline radical cation. Chemistry 19:11216–11223
Tian S, Zhang C, Huang D, Wang R, Zeng G, Yan M, Xiong W, Zhou C, Cheng M, Xue W, Yang Y, Wang W (2020) Recent progress in sustainable technologies for adsorptive and reactive removal of sulfonamides. Chem Eng J 389:123423
Tian Y, Zhou M, Pan Y, Du X, Wang Q (2021) MoS2 as highly efficient co-catalyst enhancing the performance of Fe0 based electro-Fenton process in degradation of sulfamethazine: approach and mechanism. Chem Eng J 403:126361
Veitch NC (2004) Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry 65:249–259
Wang J, Zhuan R (2020) Degradation of antibiotics by advanced oxidation processes: An overview. Sci Total Environ 701:135023
Wang B, Lu J, Dubey KD, Dong G, Lai W, Shaik S (2016) How do enzymes utilize reactive OH radicals? Lessons from nonheme HppE and Fenton systems. J Am Chem Soc 138:8489–8496
Wang T, Zhou Y, Cao S, Lu J, Zhou Y (2019) Degradation of sulfanilamide by Fenton-like reaction and optimization using response surface methodology. Ecotoxicol Environ Saf 172:334–340
Weng SS, Ku KL, Lai HT (2012) The implication of mediators for enhancement of laccase oxidation of sulfonamide antibiotics. Bioresour Technol 113:259–264
Weng SS, Liu SM, Lai HT (2013) Application parameters of laccase-mediator systems for treatment of sulfonamide antibiotics. Bioresour Technol 141:152–159
Yang L, Shi Y, Li J, Fang L, Luan T (2018) Transformation of aqueous sulfonamides under horseradish peroxidase and characterization of sulfur dioxide extrusion products from sulfadiazine. Chemosphere 200:164–172
Yassin MA, Gad AAM (2020) Immobilized enzyme on modified polystyrene foam waste: a biocatalyst for wastewater decolorization. J Environ Chem Eng 8:104435
Zhang R, Liu Y, Huang X, Xu M, Liu R, Zong W (2018) Interaction of a digestive protease, Candida rugosa lipase, with three surfactants investigated by spectroscopy, molecular docking and enzyme activity assay. Sci Total Environ 622-623:306–315
Zhang T, Cai L, Xu B, Li X, Qiu W, Fu C, Zheng C (2019a) Sulfadiazine biodegradation by Phanerochaete chrysosporium: mechanism and degradation product identification. Chemosphere 237:124418
Zhang X, Li C, Pan J, Liu R, Cao Z (2019b) Searching for a bisphenol A substitute: effects of bisphenols on catalase molecules and human red blood cells. Sci Total Environ 669:112–119
Funding
This study has been supported by the National Natural Science Foundation of China (42077391) and Shandong Provincial Natural Science Foundation, China (ZR2019BB049).
Author information
Authors and Affiliations
Contributions
Hong Liu: writing—original draft, data curation, investigation, methodology; Zaihui Huang: investigation, writing—original draft, formal analysis; Chunguang Liu: experiment design, data analysis, and writing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. HRP-Fenton-like system is developed to degrade SMR by adding Fe2+ to the HRP system.
2. Trace Fe2+ (≤ 10 μM/mL) addition can increased SMR removal by 20.7–42% at pH 5.
3. HRP, not Fenton, dominated SMR removal in the HRP-Fenton-like system.
4. Cl−/HCO3− inhibits Fenton, but has little effect on HRP-Fenton-like for SMR removal.
Rights and permissions
About this article
Cite this article
Liu, H., Huang, Z. & Liu, C. Development of a horseradish peroxidase-Fenton-like system for the degradation of sulfamethazine under weak acid condition. Environ Sci Pollut Res 29, 12065–12074 (2022). https://doi.org/10.1007/s11356-021-16681-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16681-6