Abstract
Glucocorticoids (GCs) have drawn great concern due to widespread contamination in the environment and application in treating COVID-19. Most studies on GC removal mainly focused on aquatic environment, while GC behaviors in soil were less mentioned. In this study, degradation of three selected GCs in soil has been investigated using citric acid (CA)–modified Fenton-like processes (H2O2/Fe(III)/CA and CaO2/Fe(III)/CA treatments). The results showed that GCs in soil can be removed by modified Fenton-like processes (removal efficiency gt; 70% for 24 h). CaO2/Fe(III)/CA was more efficient than H2O2/Fe(III)/CA at low oxidant dosage (< 0.28–0.69 mmol/g) for long treatment time (> 4 h). Besides the chemical assessment with GC removal, effects of Fenton-like processes were also evaluated by biological assessments with bacteria and plants. CaO2/Fe(III)/CA was less harmful to the richness and diversity of microorganisms in soil compared to H2O2/Fe(III)/CA. Weaker phytotoxic effects were observed on GC-contaminated soil treated by CaO2/Fe(III)/CA than H2O2/Fe(III)/CA. This study, therefore, recommends CaO2-based treatments to remediate GC-contaminated soils.
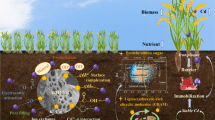
Graphical abstract

Similar content being viewed by others
Introduction
Pharmaceuticals and pharmaceutical byproducts have since been identified as one of the major groups of the emerging environmental pollutants and are present in different segments of the environment, including soils (Wu and Bi 2019). Synthetic glucocorticoids (GCs) are a class of steroid hormones which could potentially disturb the corticosteroid signaling pathways both in wildlife and humans (Wu et al. 2019), and are among the most prescribed drugs in the world against allergies, autoimmune, and inflammatory disorders (Banaschik et al. 2015). Moreover, GCs are urgently used to treat patients infected by the 2019 novel coronavirus (COVID-19) (Russell et al. 2020). Due to the vast usage, GCs have been identified as a group of emerging environmental pollutants which are present in different segments of the environment (Jia et al. 2016; Schriks et al. 2010; Suzuki et al. 2015). Exposure to exogenous GCs has been associated with health complications such as obesity, osteoporosis, cardiovascular disease, impaired development, inflammations, type-2-diabetes, and autoimmune diseases (Schriks et al. 2010). Therefore, it is imperative and urgent to control GC pollution in the environment.
Fluocinolone acetonide (FA), triamcinolone acetonide (TA), and clobetasol propionate (CP) are among the principal contributors to activities of GCs in the environment (Jia et al. 2016). However, they have been reported to be relatively stable and difficult to remove completely by traditional wastewater treatment plants (WWTPs) (Weizel et al. 2018). Piram et al. (2008) even found higher concentrations of FA in effluents compared to influents of a WWTP attributing to GC conjugate hydrolysis. Therefore, these GCs can be released into the soil through untreated wastewater, WWTP effluents, waste activated sludge, pharmaceutical residues, and animal manures (Abdellah et al. 2020), causing soils to become significant reservoirs for GCs (Shargil et al. 2016). To make matters worse, the GCs accumulated in soil may enter the food chain (Shargil et al. 2016). Shargil et al. (2016) studied GC occurrence in lettuce plants and found that corticosterone was present in the biosolids (11–92 ng/g) and lettuce plants (1–900 ng/g), indicating a cumulative tendency of GCs in plants. It is therefore, imperative to reduce these pollutants from the soil so as to guarantee safety for both humans and ecological systems.
As a group of non-biodegradable organic pollutants (Weizel et al. 2018), FA, TA, and CP can be degraded by ·OH radicals during advanced oxidation processes (AOPs) (Zhang et al. 2018a; Zhang et al. 2018b). As a typical representative of AOPs, Fenton processes are widely used in the remediation of contaminated soils due to their cost-effectiveness and practical viabilities (Hwang and Kim 2021; Zhou et al. 2017). However, conventional Fenton process is only limited for narrow pH (pH = 2–4) because iron ions will be precipitated at higher pH values (Li and Zhang 2014). To overcome this limitation, researchers have modified the conventional Fenton process by using Fe(III) and chelating agents such as citric acid (CA) to replace Fe(II) as shown in Eqs. 1–4 (Hwang and Kim 2021; Miao et al. 2015; Zhang et al. 2017). In this modified Fenton system, the use of Fe(III) and chelating agents enable the generation of ·OH radicals occurred under neutral conditions (Miao et al. 2015), which could maintain soil functions and decrease the chemical costs of soil remediation.
Besides the narrow pH limitation, another limitation for the application of conventional Fenton process is the storing and transport of hydrogen peroxide (H2O2). Since H2O2 is unstable and has a short life span in soil, alternative oxidants have been considered for soil remediation (Zhang et al. 2018a). As a potential replacement for liquid H2O2, calcium peroxide (CaO2) is widely used in water treatment, sediment, and soil remediation (Ndjou’ou and Cassidy 2006). CaO2 dissolves in water forming H2O2 in a wide pH range and over a relatively longer period of time in soil (weeks to months) (Eq. 5), achieving higher pollutant removal rate than liquid H2O2 without introducing additional toxic chemicals (Bogan and Trbovic 2003; Ndjou’ou and Cassidy 2006; Northup and Cassidy 2008; Zhang et al. 2015). Our previous research found that CaO2 is effective in GC degradation in aqueous solutions due to its synergistic effects in oxidation, adsorption, and base catalysis (Zhang et al. 2018a). Moreover, CaO2 is a slow oxygen-releasing reagent which can promote the micro-biological mineralization of pollutants in soil in the long term (Lu et al. 2017). In addition, Fe(III) was identified as a more appropriate catalyst than Fe(II) due to the slower reaction rate of Fe(III) with H2O2, which better matches the dissolution rate of CaO2 (Gan et al. 2018). Therefore, CA-modified Fe(III) catalyzed CaO2 remediation has significant potential.
Though chemical remediation by modified Fenton oxidation may be effective for GC removal in soil, the associated effects on terrestrial organisms and plant growth after GC removal are largely unknown. After soil remediation by modified Fenton oxidation, the residual chemicals may cause ecological toxicity because they change the soil properties and microorganisms (Long et al. 2016; Ping et al. 2018). Additionally, some studies indicated that free radicals and oxidizing species produced during AOPs may be harmful to plant species (Abdul et al. 2012). To our knowledge, most studies evaluated the efficiency of oxidation process in soil remediation only by chemical analysis of pollutant removal; however, studies describing the biological toxicity remain incomplete.
We therefore determined whether the modified Fenton-like oxidations (H2O2/Fe(III)/CA treatment and CaO2/Fe(III)/CA treatment) are effective in GC removal in soil and evaluated their influences on the treated soils using both chemical and biological assessments. Effects of H2O2/Fe(III)/CA and CaO2/Fe(III)/CA treatments on bacteria in soil and subsequent plant growth were investigated and comparisons drawn. This study, therefore, provides reference for the development of modified Fenton-like remediation technologies for soils contaminated with pharmaceuticals, particularly the GCs.
Materials and methods
Chemicals, soils, and seeds
The three GC standards (FA, CAS: 67-73-2; TA, CAS: 76-25-5; CP, CAS: 25122-46-7) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Their chemical structures were as shown in Fig. 1. CaO2 regent (75%, 80–150 mesh) was purchased from Aladdin Reagent Co. Ltd. (Shanghai, China). Methanol, acetonitrile, and methyl tert-butyl ether (MTBE) were of chromatographic grade and obtained from Sigma-Aldrich. Hydrogen peroxide (H2O2, 30% (w/v)), citric acid (2-hydroxy-1,2,3-propanetricarboxylic acid, CA, purity > 99.5%), and iron (III) chloride hexahydrate (Fe(III)) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) was obtained from Sigma-Aldrich (St. Louis, USA). Other chemicals were of analytical grade and purchased from Macklin Inc. (Shanghai, China). Ultrapure water was collected from a Barnstead Ultra-Pure instrument (EMD Millipore, Billerica, MA, USA).
A silty clay soil was collected from Donghua University Songjiang campus (N 31°3′30″; E 121°12′18″) and air-dried and sieved through a 2-mm mesh before use. The main characteristics (average data) of the soil were as follows: sand grains (> 20 μm) 14.0%, silt grains (2-20 μm) 57.2%, clay grains (< 2 μm) 28.8%, soil pH 7.45, moisture content 1.44%, organic content 3.22%, soil conductivity 402 μS/cm, redox potential, 119 mV. Pea seeds were purchased from a local agricultural market and stored at 4 °C before use. Each 300 seeds weighed between 150 and 180 g.
Experimental procedure
The performance of H2O2/Fe(III)/CA and CaO2/Fe(III)/CA on FA, TA, and CP removal from soils were separately evaluated. The GC polluted soil slurry was prepared by mixing 10 g of soil with 50 mL of GC solution (prepared by spiking concentrated GC solution into deionized water) on a mechanical shaker overnight. Considering the environmental concentration of GCs (Table S1 in supporting information (SI)) and their widespread use during COVID-19, the initial GC concentration in soil solution was 5 μmol/g dry soil. Appropriate CA and Fe(III) solutions were spiked into the GC-polluted soil slurry and then preset amount of oxidant (H2O2 or CaO2) was added into the GC-polluted soil slurry to start the experiment while magnetically stirred. Effects of oxidant (H2O2 or CaO2) type and treatment time (0–24 h) on GC removal were evaluated by adding 0.69 mmol/g dw H2O2 or CaO2 into GC-polluted soil slurries with molar ratio of oxidant (H2O2 or CaO2):Fe(III):CA fixed at 4:1:2. Effects of oxidant dosage (0–1.39 mmol/g dw) were evaluated with molar ratio of oxidant:Fe(III):CA fixed at 4:1:2 for 4 h. Effects of CA and Fe(III) dosages on GC removal during CaO2/Fe(III)/CA oxidation were investigated at various molar ratios of CaO2:Fe(III):CA (20:1:2, 20:2:4, 20:5:10, 20:7.5:15, and 20:10:20) at fixed CaO2 dosage (1.11 mmol/g dw) for 4 h (the oxidant dosages and ratio-values were chosen based on our pre-experiments with data not shown). Detailed experimental design was as shown in Table S2 in SI. For the blank, same volume of deionized water was spiked into soil slurry without GC addition and with no CA, Fe(III), and oxidant added. For the control, same volume of deionized water was spiked into the GC-polluted soil slurry with no CA and Fe(III) solution and oxidant added. All the experiments were conducted under dark conditions. To study the effects of H2O2/Fe(III)/CA and CaO2/Fe(III)/CA on soil indigenous microorganisms and phytotoxicity, experiments were performed at oxidant dosage (H2O2 or CaO2) of 1.11 mmol/g dw and molar ratio of oxidant (H2O2 or CaO2):Fe(III):CA at 4:1:2 with pH unadjusted. For the blank, same volume of deionized water was spiked into soil slurry without GC addition and with no CA and Fe(III) solution and oxidant added. Ten milliliters of soil slurry samples was taken at the preset sampling times with sodium thiosulfate (Na2S2O3) as the terminator and analyzed immediately.
Analysis
GC extraction and analysis
The soil sample preparation procedure for GCs followed the method of our previous research (Zhang and Li 2014). Briefly, soil samples were freeze-dried and extracted with methanol and acetate buffer solution (pH 5.0) mixture (90:10, v/v) by ultrasonic. The extracts were evaporated in a rotatory evaporator and extracted with solid-phase extraction (SPE) according to Jia et al. (2016). Detailed analysis method can be found in Text S1 in SI. The extracts of SPE were then concentrated with nitrogen to a final volume of 5 mL for ultra-high-performance liquid chromatography (UHPLC, Dionex UltiMate 3000, Thermo Fisher Scientific Inc., USA) analysis. Detailed methods for UHPLC were as reported in our previous researches (Zhang et al. 2018a; Zhang et al. 2018b) and shown in Text S2 in SI. Under these experimental conditions, the quantification limits of FA, TA, and CP were 1.82, 1.63, and 1.47 nmol/g dw, respectively. The recoveries for FA, TA, and CP were in the range of 85–91%, 83–89%, and 94–102%, respectively.
Molecular analysis of microbial communities in soil
To evaluate the individual influence of H2O2/Fe(III)/CA and CaO2/Fe(III)/CA on microorganisms, the bacterial communities in soil samples after H2O2/Fe(III)/CA and CaO2/Fe(III)/CA treatments were analyzed. The procedure was performed according to a previous report (Ping et al. 2018). Detailed information was as described in Text S3 in SI. The raw sequences data have been deposited with the accession number SRP198736 (https://www.ncbi.nlm.nih.gov/Traces/sra_sub/).
Seed germination analysis
Seed germination experiments were conducted to detect the phytotoxic effects of GC polluted soil before and after H2O2/Fe(III)/CA and CaO2/Fe(III)/CA separate treatments (Gao et al. 2019; Jia et al. 2018). Fifty milliliters of soil slurry samples was collected before (the “blank”) and after GC polluted (the “control”) and after H2O2/Fe(III)/CA treatment or CaO2/Fe(III)/CA treatment. Then 50 pea seeds were uniformly put into the soil sample in a seedbed (plastic mesh with each hole containing a seed) in a petri dish (Φ 140 mm) (Gao et al. 2019; Jaouani et al. 2018). The dish was placed inside a germination chamber equipped with daylight fluorescent lamps. After 6 days of germination, germination rate, shoot length, and dry weight were measured as described in Text S4 in SI. The significance test of difference was analyzed with SPSS statistical software (Version 16, SPSS Inc., Chicago, IL, USA).
Other analysis
Electron paramagnetic resonance spectroscopy (EPR) was used for detection of free radicals during CaO2/Fe(III) and CaO2/Fe(III)/CA treatments as described in Text S5 in SI. Oxidation reduction potential (ORP) and pH was recorded in situ using a multi-probe instrument (HANNA instrument HI9829, Italy). H2O2 concentration was analyzed by potassium titanium (IV) oxalate using a spectrophotometer (TU-1810, China) at 387 nm (Gao et al. 2019).
Results and discussion
GC degradation performance by oxidant/Fe(III)/CA treatments in soil
Effects of oxidant dosage
The results of GC removal at varying treatment times (0–24 h) with oxidant dosage of 0.69 mmol/g dw and molar ratio of oxidant:Fe(III):CA = 4:1:2 were as shown in Fig. 2. GC removal efficiency increased with the increase of treatment time from 0 to 24 h. At treatment time of 24 h, the GC removal efficiencies were 70–77% and 78–89% for H2O2/Fe(III)/CA and CaO2/Fe(III)/CA treatment, respectively, indicating that GCs in soil can be removed by modified Fenton-like processes.
The removal efficiency of FA, TA, and CP during H2O2/Fe(III)/CA treatment reached 74%, 70%, and 77% at 24 h, respectively. In comparison, the removal efficiency of FA, TA, and CP during CaO2/Fe(III)/CA treatment reached 78%, 78%, and 89% at 24 h, respectively (Fig. 2). At treatment time of 12h for FA, 8h for TA, and 4h for CP, reactions with CaO2/Fe(III)/CA treatment began to show higher GC removal efficiencies than those with H2O2/Fe(III)/CA treatment (Fig. 2). CaO2 can release H2O2 continuously and slowly upon dissolution (Eq. 5), thus reducing the loss of H2O2 to O2 (Eq. 6) and volatilization, achieving a greater oxidant efficiency than liquid H2O2 (Zhang et al. 2015).
After 4 h, the increasing tendency of GC removal efficiency slowed down for both H2O2/Fe(III)/CA and CaO2/Fe(III)/CA treatments (Fig. 2). At treatment time of 24 h, the removal efficiencies for all target GCs were greater than 50% (Fig. 2). Considering both the removal of GCs and the time efficiency, treatment time of 4h was chosen for the following experiments.
Effects of oxidant dosage
The results of GC removal at varying oxidant concentrations (0–1.39 mmol/g dw) with the molar ratio of oxidant:Fe(III):CA = 4:1:2 at 4 h were as shown in Fig. 3. The GC removal rate increased with the increase of oxidant concentrations from 0 to 1.39 mmol/g except for the case of CP at H2O2 dosage of 1.39 mmol/g (Fig. 3). Substantive increments in H2O2 and CaO2 dosages increased the generation of reactive radicals (such as ·OH) as shown in Eqs. 1–5 (Hu et al. 2019), thus improving the GC degradation.
At oxidant dosages of 0.28 mmol/g for FA and 0.28–0.69 mmol/g for CP, reactions with CaO2/Fe(III)/CA treatment showed higher GC removal efficiencies than those with H2O2/Fe(III)/CA treatment (Fig. 3). Similar results were also obtained by Bogan and Trbovic (2003) and Ndjou’ou and Cassidy (2006) in treating polycyclic aromatic hydrocarbon (PAH)–contaminated soil and total petroleum hydrocarbon (TPH)–contaminated soil by CaO2 and H2O2, respectively. In conditions with insufficient oxidants, CaO2 was more effective than H2O2 in removing organic pollutants (Zhang et al. 2015). However, at high oxidant concentrations of 1.11–1.39 mmol/g dw, H2O2/Fe(III)/CA treatment performed better than CaO2/Fe(III)/CA in GC removal at 4 h (Fig. 3). Northup and Cassidy (2008) reported that complete dissolution of unbuffered CaO2 required 62 days. Although H2O2-based treatment achieved higher GC removal efficiencies than CaO2-based treatment in a short time (4 h), CaO2 may achieve better removal efficiencies in a long time because of its consistent releasing of H2O2 and ·OH.
It is worth noting that there was a comparative decrease (14%) for CP degradation when H2O2 dosage increased from 1.11 to 1.39 mmol/g (Fig. 3c). This could be attributed to the reactive effects of radical scavengers as shown in Eqs. 7–9 (Lemaire et al. 2013). When H2O2 was in excess, it became a scavenger for ·OH (Eqs. 7–9), thus inhibiting oxidation of CP (Seol and Javandel 2008). Since the O/C ratio of CP is 20% lower than that of TA and FA and the H/C ratio of CP is 7% and 4% lower than that of TA and FA respectively (Fig. 1), the oxidation state of CP was the lowest among the target GCs (Liu et al. 2019a, 2019b), causing CP the most sensitive one to ·OH concentration among the three target GCs.
As the increasing tendency of GC removal rate slowed down with CaO2 dosage increase from 1.11 to 1.39 mmol/g, the overall effect on GC removal was limited (≤ 5%). This could be due to the fact that increasing CaO2 dosage increased the soil pH by releasing Ca(OH)2 (Eq. 5). In alkaline conditions, the Fe(III) precipitates and H2O2 decomposes into oxygen without generating ·OH (Seol and Javandel 2008). The removal rate of FA, TA, and CP reached 60%, 54%, and 57%, respectively, at 4 h with CaO2 dosage of 1.11 mmol/g. Similar magnitudes of organic pollutant degradation were also observed in Fe(II), Fe(III) and ascorbic acid, CA-activated CaO2, and H2O2 systems. Zhang et al. (2017) reported that the removal rate of nitrobenzene and carbon tetrachloride in water was 59% and 15%, respectively, with CaO2/Fe(III)/ascorbic acid treatment for 90 min. Lemaire et al. (2013) reported an oxidation ratio of 45% for PAHs in soil with H2O2/Fe(II)/CA treatment.
Effects of CA and Fe(III) dosages
To investigate the effect of CA on activating CaO2/Fe(III)/CA for GC-contaminated soil remediation, the GC removal efficiencies at various CA dosages were evaluated as shown in Fig. 4. The GC removal rate was below 25% in CaO2/Fe(III) system at various Fe(III) dosages without CA addition (Fig. 4a). The addition of CA to CaO2/Fe(III) system improved GC removal significantly (Fig. 4b). In absence of CA, the soil pH increased with CaO2 addition. At alkaline conditions, Fe(III) precipitated quickly (Zhang et al. 2017), thus decreasing the generation rate of ·OH radicals. Conversely, with CA addition, the concentration of Fe(III) could be maintained as the soil pH decreased and the Fe(III)-CA complex formed (Zhang et al. 2017), promoting the generation rate of ·OH radicals and thus improved GC removal efficiency. As shown in Fig. S1, both ·OH and ·O2− were detected in CaO2-based systems and the CaO2/Fe(III)/CA system achieved more significant ·OH radical signals than CaO2/Fe(III) system. Miao et al. (2015) reported that the chelators could effectively enhance the removal of tetrachloroethene during percarbonate/Fe(III) treatment by chelating with Fe(III). Moreover, the acidic pH caused by adding CA would neutralize the alkalinity from CaO2 and accelerate H2O2 release and ·OH generation during CaO2 dissolution (Wang et al. 2016). Wolanov et al. (2013) reported that the dissolution of CaO2 would decrease to zero with pH rising to 11. Arienzo (2000) detected the pH dependency of the H2O2 concentration in CaO2 slurry and found that acidic condition could accelerate the release of H2O2 from CaO2. To further confirm the effect of acidification and acceleration of H2O2 or ·OH generation by CA during CaO2/Fe(III)/CA treatment, changes in pH and H2O2 concentrations during the treatment were investigated and the EPR analysis was used. As shown in Fig. 4 c and d, with the increase of CA dosage, the solution pH decreased and H2O2 concentration increased. The EPR spectrum which has four split lines with 1:2:2:1 height ratio and g = 2.005 at the symmetry center represented the formation of ·OH radicals during CaO2/Fe(III)/CA treatment (Fig. 4d).
The removal efficiencies of GCs a by CaO2/Fe(III) treatment at varying Fe(III) concentrations and b by CaO2/Fe(III)/CA treatment at varying Fe(III) and CA concentrations; c H2O2 concentration and pH value at 30 min at various mole ratios of CaO2:Fe(III):CA; d EPR spectrum of radicals released during CaO2/Fe(III)/CA treatment (CaO2 dosage = 1.11 mmol/g dw)
In addition, the GC removal rate increased with the increase of CA and Fe(III) except for CP removal at CaO2:Fe(III):CA = 20:10:20 (Fig. 4b). Decreasing removal rate of CP with increasing CA concentration was observed at CaO2:CA < 4:3. Possible explanation for this declining removal efficiency of CP could be the scavenging effects of CA to ·OH radicals. CA is a competitor for ·OH radical and part of the oxidant may be consumed by CA (Zhou et al. 2017). In the present case, the high content of CA (CaO2:CA < 4:3) may have consumed a large part of the oxidant and ·OH radical, causing the decrease of CP removal efficiency. For FA and TA, they belong to acetonide, while for CP, it belongs to ester (Fig. 1). Compared to esters, acetonides are less labile (Wiedersberg et al. 2008). Therefore, the FA and TA were not influenced by the overdosed CA.
Effects of oxidant/Fe(III)/CA treatment on indigenous microorganisms in soil
Microbial samples were taken from the control, H2O2/Fe(III)/CA, and CaO2/Fe(III)/CA treatments conducted at 4 h. Effective sequences of 890,856 were achieved from 9 samples with the minimum number of 38,745 from each sample. Through clustering, a total of 3,212 operational taxonomic units (OTUs) were recovered at the cutoff level of 3%. These data were sufficient enough to ensure the accuracy of the rarefaction analysis (Fig. S2).
Microbial richness and diversity
To illustrate the community diversity and sample coverage, the alpha-diversity indices were calculated and listed in Table 1. All Good’s coverages from these samples were beyond 99%, reflecting a credible presentation (Shi et al. 2018). According to Table 1, the samples without oxidation (control) showed higher microbial richness and more microorganism species than the samples with either H2O2/Fe(III)/CA or CaO2/Fe(III)/CA treatments. This could be largely associated with the sensitivity of microorganisms to oxidants (Ping et al. 2018). As an oxidant, the ·OH radical released by H2O2 and CaO2 directly attacks the polyunsaturated fatty acids in microbial membranes (Ping et al. 2018). This initiates lipid peroxidation, which kills or inactivates some microorganisms in soil (Ping et al. 2018), thus lowering the richness and diversity of microorganisms in the soil. Moreover, the richness and diversity of microorganism species after H2O2/Fe(III)/CA treatment were much lower than those after CaO2/Fe(III)/CA treatment (Table 1). Contrary to H2O2 which releases high levels of ·OH radicals in a short time, the solid CaO2 could release H2O2 and ·OH radical upon dissolution (Eq. 5) and maintain lower levels of H2O2, ·OH radical, and O2 (Eq. 6) over a relatively longer period as noted earlier (“Effects of oxidant dosage” section), thus minimizing the sudden shock to microorganisms. Additionally, CaO2/Fe(III)/CA treatment is more compatible with microbial reactions than H2O2/Fe(III)/CA treatment as the operating pH is near neutral (pH = 7.9 at CaO2/Fe(III)/CA = 20:5:10). Based on the above results, CaO2/Fe(III)/CA treatment is, therefore, a preferred approach for contaminated soil remediation as it is favorable to the microorganisms compared to H2O2/Fe(III)/CA treatment.
Microbial composition
According to the Venn diagram (Fig. S3), OTUs implied that only 193 OTUs from a total of 3,212 were shared by the three treatment approaches, which implied that microbial communities in control, H2O2/Fe(III)/CA-, and CaO2/Fe(III)/CA-treated samples were different.
The dominant distributions of phylum and genus level are depicted in Fig. 5 and Fig. S4, respectively. In phylum level, Proteobacteria (control 41%, H2O2/Fe(III)/CA 25%, CaO2/Fe(III)/CA 22%), Firmicutes (control 0.5%, H2O2/Fe(III)/CA 30%, CaO2/Fe(III)/CA 29%), Bacteroidetes (control 9%, H2O2/Fe(III)/CA 15%, CaO2/Fe(III)/CA 16%), and Actinobacteria (control 24%, H2O2/Fe(III)/CA 6%, CaO2/Fe(III)/CA 10%) were predominant (Fig. 5). In genus level, the dominant distributions were Nitrosomonadaceae (5%) for the control and Peptostreptococcaceae (H2O2/Fe(III)/CA 9%, CaO2/Fe(III)/CA 9%) for oxidized samples (Fig. S4).
After H2O2/Fe(III)/CA or CaO2/Fe(III)/CA treatment, the proportion of Proteobacteria, Actinobacteria, and Acidobacteria decreased while the percent of Firmicutes and Bacteroidetes increased. The lipid and humic-like substances in soil greatly affected the distribution of phylum Proteobacteria while the aromatic and humic-like substances affected the phylum Actinobacteria (Liu et al. 2019a, 2019b). The percent decrease of Proteobacteria and Actinobacteria after H2O2/Fe(III)/CA or CaO2/Fe(III)/CA treatment indicated the content decrease of the lipid, aromatic, and humic-like substances in soil after oxidation. Bacteroidetes was important in organic matter degradation and the carbon cycle (Newton et al. 2011) and Firmicutes was important in the synthesis and humification of fulvic-like substances (Liu et al. 2019a, 2019b). Besides, Firmicutes and Bacteroidetes can release extracellular enzymes to promote the degradation and hydrolysis of semi-cellulose, cellulose, and proteins (Kong et al. 2018). This implies that the proportionate increase in Firmicutes and Bacteroidetes enhanced hydrolysis, degradation of organic matter, and humification after H2O2/Fe(III)/CA or CaO2/Fe(III)/CA treatment.
Phytotoxic effects of oxidant/Fe(III)/CA treatment
The phytotoxicity of H2O2/Fe(III)/CA and CaO2/Fe(III)/CA treatments on GC-contaminated soil was evaluated by monitoring germination rate, shoot length, and plant dry weight of pea. As depicted in Fig. 6, the pea seeds in the control (non-treated GC-contaminated soil) had lower germination rate (7% reduction), shoot length (41% reduction), and plant dry weight (41% reduction) compared to those in the blank (uncontaminated soil), demonstrating the strong toxicity effect of GCs to plants. After the application of H2O2/Fe(III)/CA treatment on the GC-contaminated soil, a greater negative impact on the germination (33% reduction), shoot length (31% reduction), and plant dry weight (64% reduction) of pea seeds was observed compared to those in control (Fig. 6). This meant that H2O2/Fe(III)/CA treatment increased the phytotoxic effects of the GC-contaminated soil. In contrast, CaO2/Fe(III)/CA treatment showed much weaker phytotoxic effects to pea seeds than with similar germination rate and plant dry weight before and after treatment (Fig. 6). A greater shoot length (7.08 cm) was found after CaO2/Fe(III)/CA treatment of the GC-contaminated soil (5.67cm in control, Fig. 6). As shown in Table S3, the ORP for GC-contaminated soil increased from 116 to 559 mV after H2O2/Fe(III)/CA treatment. Comparatively, the ORP changed slightly (from 116 to 121 mV) after CaO2/Fe(III)/CA treatment, indicating that the oxidative stress caused by CaO2 was much weaker than that caused by H2O2. Furthermore, the release of Ca2+ from CaO2 during CaO2/Fe(III)/CA treatment may also contribute to the germination and growth of pea seeds. It has been well established that Ca2+ and calmodulin are key regulatory elements in many cellular processes in animals and plants (Corpas and Barroso 2018). The presence of Ca2+ also closely correlates with that of Ca2+-dependent protein kinase predominantly involved in lipid metabolism, oxidative stress, mediating pathogen resistance, and pollen tube growth (Corpas and Barroso 2018). Since H2O2/Fe(III)/CA treatment was more phytotoxic on terrestrial plants, CaO2/Fe(III)/CA treatment was recommended to remove GCs from the contaminated soil and simultaneously reduce the phytotoxicity to terrestrial plants.
Phytotoxicity of soil samples on seeding germination, shoot length, and plant dry weight of pea of the blank, control, H2O2/Fe(III)/CA, and CaO2/Fe(III)/CA treatments. The letter above the bar indicates significant differences on shoot length among the blank and the control at p < 0.01. The double asterisk (**) indicates p < 0.01 for a Student t test carried out on shoot length between the control and oxidant/Fe(III)/CA treatments (oxidant concentration = 1.11 mmol/g dw, Fe(III) = 0.28 mmol/g dw, CA = 0.56 mmol/g dw)
Conclusions
H2O2/Fe(III)/CA and CaO2/Fe(III)/CA treatments were effective for GC removal in soils. Both H2O2 and CaO2 systems achieved over 53% of GC removal efficiencies at oxidant dosage of 1.11 mmol/g dw with the molar ratio of oxidant:Fe(III):CA = 4:1:2 at 4 h. CaO2/Fe(III)/CA could be more efficient than H2O2/Fe(III)/CA for GC removal at low oxidant dosages (< 0.28–0.69 mmol/g) for long treatment times (> 4 h).
H2O2/Fe(III)/CA and CaO2/Fe(III)/CA treatments had significant influences on bacteria in treated soils and subsequent plant growth. The richness and number of microorganism species decreased and the microbial communities changed after H2O2/Fe(III)/CA and CaO2/Fe(III)/CA treatments. Compared to H2O2/Fe(III)/CA treatment, CaO2/Fe(III)/CA treatment was less harmful to the richness and diversity of microorganisms in soil. Weaker phytotoxic effects were observed on GC-contaminated soil treated by CaO2/Fe(III)/CA than H2O2/Fe(III)/CA.
Considering both GC removal and subsequent utilization of soils, CaO2/Fe(III)/CA treatments are recommended for remediation of soils contaminated with GCs or other organic pollutants.
Data availability
The authors declare that all relevant data supporting the findings of this study are included in this article and its supplementary information files.
References
Abdellah YAY, Zang H, Li C (2020) Steroidal estrogens during composting of animal manure: persistence, degradation, and fate, a review. Water Air Soil Pollut 231:547–447. https://doi.org/10.1016/j.jhazmat.2016.11.009
Abdul JM, Colville A, Lim R, Vigneswaran S, Kandasamy J (2012) Use of duckweed (Lemna disperma) to assess the phytotoxicity of the products of Fenton oxidation of metsulfuron methyl. Ecotox Environ Safe 83:89–95. https://doi.org/10.1016/j.ecoenv.2012.06.014
Arienzo M (2000) Degradation of 2, 4, 6-trinitrotoluene in water and soil slurry utilizing a calcium peroxide compound. Chemosphere 40:331–337. https://doi.org/10.1016/s0045-6535(99)00212-x
Banaschik R, Lukes P, Jablonowski H, Hammer MU, Weltmann KD, Kolb JF (2015) Potential of pulsed corona discharges generated in water for the degradation of persistent pharmaceutical residues. Water Res 84:127–135. https://doi.org/10.1016/j.watres.2015.07.018
Bogan BW, Trbovic V (2003) Effect of sequestration on PAH degradability with Fenton’s reagent: roles of total organic carbon, humin, and soil porosity. J Hazard Mater 100:285–300. https://doi.org/10.1016/s0304-3894(03)00134-1
Corpas FJ, Barroso JB (2018) Peroxisomal plant metabolism - an update on nitric oxide, Ca2+ and the NADPH recycling network. J Cell Sci 131(2):8. https://doi.org/10.1242/jcs.202978
Gan YY, Zhou SL, Dai X, Wu H, Xiong ZY, Qin YH, Ma J, Yang L, Wu ZK, Wang TL, Wang WG, Wang CW (2018) Effect of iron salt type and dosing mode on Fenton-based pretreatment of rice straw for enzymatic hydrolysis. Bioresour Technol 265:394–398. https://doi.org/10.1016/j.biortech.2018.06.043
Gao X, Zhang A, Héroux P, Sand W, Sun Z, Zhan J, Wang C, Hao S, Li Z, Guo Y, Liu Y (2019) Effect of dielectric barrier discharge cold plasma on pea seed growth. J Agric Food Chem 67:10813–10822. https://doi.org/10.1021/acs.jafc.9b03099
Hu EZ, He Z, Nan XL, Yuan ZJ, Li XJ (2019) Removal of phenanthrene and pyrene from contaminated sandy soil using hydrogen peroxide oxidation catalyzed by basic oxygen furnace slag. Environ Sci Pollut R 26:9281–9292. https://doi.org/10.1007/s11356-019-04308-w
Hwang JI, Kim JE (2021) Removal of organochlorine insecticide endosulfan in water and soil by Fenton reaction with ascorbic acid and various iron resources. Environ Sci Pollut R 28:28479–28489. https://doi.org/10.1007/s11356-021-12439-2
Jaouani K, Karmous I, Ostrowski M, El Ferjani E, Jakubowska A, Chaoui A (2018) Cadmium effects on embryo growth of pea seeds during germination: investigation of the mechanisms of interference of the heavy metal with protein mobilization-related factors. J Plant Physiol 226:64–76. https://doi.org/10.1016/j.jplph.2018.02.009
Jia A, Wu S, Daniels KD, Snyder SA (2016) Balancing the budget: accounting for glucocorticoid bioactivity and fate during water treatment. Environ Sci Technol 50:2870–2880. https://doi.org/10.1021/acs.est.5b04893
Jia H, Cao Y, Qu G, Wang T, Guo X, Xia T (2018) Dimethyl phthalate contaminated soil remediation by dielectric barrier discharge: performance and residual toxicity. Chem Eng J 351:1076–1084. https://doi.org/10.1016/j.cej.2018.06.173
Kong X, Yu S, Xu S, Fang W, Liu J, Li H (2018) Effect of Fe0 addition on volatile fatty acids evolution on anaerobic digestion at high organic loading rates. Waste Manag 71:719–727. https://doi.org/10.1016/j.wasman.2017.03.019
Lemaire J, Buès M, Kabeche T, Hanna K, Simonno MO (2013) Oxidant selection to treat an aged PAH contaminated soil by in situ chemical oxidation. J Environ Chem Eng 1:1261–1268. https://doi.org/10.1016/j.jece.2013.09.018
Li Y, Zhang A (2014) Removal of steroid estrogens from waste activated sludge using Fenton oxidation: influencing factors and degradation intermediates. Chemosphere 105:24–30. https://doi.org/10.1016/j.chemosphere.2013.10.043
Liu Y, Wang C, Shen X, Zhang A, Yan S, Li X, Miruka AC, Wu S, Guo Y, Ognier S (2019a) Degradation of glucocorticoids in aqueous solution by dielectric barrier discharge: kinetics, mechanisms, and degradation pathways. Chem Eng J 374:412–428. https://doi.org/10.1016/j.cej.2019.05.154
Liu SJ, Xi BD, Qiu ZP, He XS, Zhang H, Dang QL, Zhao XY, Li D (2019b) Succession and diversity of microbial communities in landfills with depths and ages and its association with dissolved organic matter and heavy metals. Sci Total Environ 651:909–916. https://doi.org/10.1016/j.scitotenv.2018.09.267
Long XE, Wang J, Huang Y, Yao HY (2016) Microbial community structures and metabolic profiles response differently to physiochemical properties between three landfill cover soils. Environ Sci Pollut R 23:15483–15494. https://doi.org/10.1007/s11356-016-6681-6
Lu S, Zhang X, Xue Y (2017) Application of calcium peroxide in water and soil treatment: a review. J Hazard Mater 337:163–177. https://doi.org/10.1016/j.jhazmat.2017.04.064
Miao Z, Gu X, Lu S, Brusseau ML, Zhang X, Fu X, Danish M, Qiu Z, Sui Q (2015) Enhancement effects of chelating agents on the degradation of tetrachloroethene in Fe (III) catalyzed percarbonate system. Chem Eng J 281:286–294. https://doi.org/10.1016/j.cej.2015.06.076
Ndjou’ou AC, Cassidy D (2006) Surfactant production accompanying the modified Fenton oxidation of hydrocarbons in soil. Chemosphere 65:1610–1615. https://doi.org/10.1016/j.chemosphere.2006.03.036
Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49. https://doi.org/10.1128/mmbr.00028-10
Northup A, Cassidy D (2008) Calcium peroxide (CaO2) for use in modified Fenton chemistry. J Hazard Mater 152:1164–1170. https://doi.org/10.1016/j.jhazmat.2007.07.096
Ping Q, Lu X, Zheng M, Li Y (2018) Effect of CaO2 addition on anaerobic digestion of waste activated sludge at different temperatures and the promotion of valuable carbon source production under ambient condition. Bioresour Technol 265:247–256. https://doi.org/10.1016/j.biortech.2018.06.007
Piram A, Salvador A, Gauvrit JY, Lanteri P, Faure R (2008) Development and optimisation of a single extraction procedure for the LC/MS/MS analysis of two pharmaceutical classes residues in sewage treatment plant. Talanta 74:1463–1475. https://doi.org/10.1016/j.talanta.2007.09.038
Russell CD, Millar JE, Baillie JK (2020) Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395:473–475. https://doi.org/10.1016/S0140-6736(20)30317-2
Schriks M, van Leerdam JA, van der Linden SC, van der Burg B, van Wezel AP, de Voogt P (2010) High-resolution mass spectrometric identification and quantification of glucocorticoid compounds in various wastewaters in the Netherlands. Environ Sci Technol 44:4766–4774. https://doi.org/10.1021/es100013x
Seol Y, Javandel I (2008) Citric acid-modified Fenton’s reaction for the oxidation of chlorinated ethylenes in soil solution systems. Chemosphere 72:537–542. https://doi.org/10.1016/j.chemosphere.2008.03.052
Shargil D, Fine P, Gerstl Z, Nitsan I, Kurtzman D (2016) Impact of biosolids and wastewater effluent application to agricultural land on corticosterone content in lettuce plants. Sci Total Environ 541:742–749. https://doi.org/10.1016/j.scitotenv.2015.09.115
Shi K, Wang C, Jiang SC (2018) Quantitative microbial risk assessment of Greywater on-site reuse. Sci Total Environ 635:1507–1519. https://doi.org/10.1016/j.scitotenv.2018.04.197
Suzuki G, Sato K, Isobe T, Takigami H, Brouwer A, Nakayama K (2015) Detection of glucocorticoid receptor agonists in effluents from sewage treatment plants in Japan. Sci Total Environ 527:328–334. https://doi.org/10.1016/j.scitotenv.2015.05.008
Wang H, Zhao Y, Li T, Chen Z, Wang Y, Qin C (2016) Properties of calcium peroxide for release of hydrogen peroxide and oxygen: a kinetics study. Chem Eng J 303:450–457. https://doi.org/10.1016/j.cej.2016.05.123
Weizel A, Schlüsener MP, Dierkes G, Ternes TA (2018) Occurrence of glucocorticoids, mineralocorticoids, and progestogens in various treated wastewater, rivers, and streams. Environ Sci Technol 52:5296–5307. https://doi.org/10.1021/acs.est.7b06147
Wiedersberg S, Leopold CS, Guy RH (2008) Bioavailability and bioequivalence of topical glucocorticoids. Eur J Pharm Biopharm 68:453–466. https://doi.org/10.1016/j.ejpb.2007.08.007
Wolanov Y, Prikhodchenko PV, Medvedev AG, Pedahzur R, Lev O (2013) Zinc dioxide nanoparticulates: a hydrogen peroxide source at moderate pH. Environ Sci Technol 47:8769–8774. https://doi.org/10.1021/es4020629
Wu L, Bi E (2019) Sorption of ionic and neutral species of pharmaceuticals to loessial soil amended with biochars. Environ Sci Pollut R 26:35871–35881. https://doi.org/10.1007/s11356-019-06721-7
Wu S, Jia A, Daniels KD, Park M, Snyder SA (2019) Trace analysis of corticosteroids (CSs) in environmental waters by liquid chromatography–tandem mass spectrometry. Talanta 195:830–840. https://doi.org/10.1016/j.talanta.2018.11.113
Zhang A, Li Y (2014) Removal of phenolic endocrine disrupting compounds from waste activated sludge using UV, H2O2, and UV/H2O2 oxidation processes: effects of reaction conditions and sludge matrix. Sci Total Environ 493:307–323. https://doi.org/10.1016/j.scitotenv.2014.05.149
Zhang A, Wang J, Li YM (2015) Performance of calcium peroxide for removal of endocrine-disrupting compounds in waste activated sludge and promotion of sludge solubilization. Water Res 71:125–139. https://doi.org/10.1016/j.watres.2015.01.005
Zhang X, Gu X, Lu S, Brusseau ML, Xu M, Fu X, Qiu Z, Sui Q (2017) Application of ascorbic acid to enhance trichloroethene degradation by Fe (III)-activated calcium peroxide. Chem Eng J 325:188–198. https://doi.org/10.1016/j.cej.2017.05.004
Zhang A, Shen X, Yin X, Li X, Liu Y (2018a) Application of calcium peroxide for efficient removal of triamcinolone acetonide from aqueous solutions: mechanisms and products. Chem Eng J 345:594–603. https://doi.org/10.1016/j.cej.2018.01.104
Zhang A, Yin X, Shen X, Liu Y (2018b) Removal of fluticasone propionate and clobetasol propionate by calcium peroxide: synergistic effects of oxidation, adsorption, and base catalysis. ES Energy & Environment 1:89–98. https://doi.org/10.30919/esee8c137
Zhou Y, Fang X, Wang T, Hu Y, Lu J (2017) Chelating agents enhanced CaO2 oxidation of bisphenol A catalyzed by Fe3+ and reuse of ferric sludge as a source of catalyst. Chem Eng J 313:638–645. https://doi.org/10.1016/j.cej.2016.09.111
Acknowledgements
All the financial supports are gratefully acknowledged.
Abbreviations
Not applicable.
Funding
This work was completed by the financial support of the National Natural Science Foundation of China (51708096, 51578122), the Shanghai Chen-Guang Program (19CG38), and the Shanghai Sailing Program (18YF1407500).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo Torres-Palma
CRediT author statement
Yanan Liu: writing—original draft; methodology; resources; Quan Zhou: methodology, formal analysis, investigation, resources, data Curation; Zhenyu Li: formal analysis, investigation, resources; Ai Zhang: writing—reviewing and editing; supervision; project administration; funding acquisition conceptualization; Jiaxun Zhan: methodology, resources, data curation; Andere Clement Miruka: investigation, writing—reviewing and editing; Xiaoting Gao: methodology, formal analysis; Jie Wang: writing—reviewing and editing.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• H2O2- and CaO2-based oxidation can effectively remove GCs from soils.
• CaO2-based oxidation was more efficient than H2O2 at low oxidant dosage.
• CaO2-based oxidation was less harmful to soil microorganisms than H2O2.
• H2O2-based oxidation showed stronger phytotoxic effect than CaO2.
Supplementary Information
ESM 1
(DOCX 200 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Zhou, Q., Li, Z. et al. Effectiveness of chelating agent–assisted Fenton-like processes on remediation of glucocorticoid-contaminated soil using chemical and biological assessment: performance comparison of CaO2 and H2O2. Environ Sci Pollut Res 28, 67310–67320 (2021). https://doi.org/10.1007/s11356-021-15150-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15150-4