Abstract
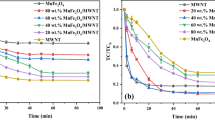
Magnetic nanostructured MnFe2O4 with different morphologies, synthesized via chemical co-precipitation and hydrothermal method, was assayed as heterogeneous Fenton catalysts. The as-prepared MnFe2O4 catalysts were thoroughly characterized by various characterization methods, such as X-ray diffraction (XRD), N2 adsorption-desorption, transmission electron microscopy (TEM), magnetic hysteresis loops, temperature-programmed reduction (TPR), and X-ray photoelectron spectroscopy (XPS). The catalytic activity of MnFe2O4 catalysts was evaluated in the heterogeneous Fenton degradation of ofloxacin (OFX). In our study, the morphology exhibited a critical impact on the catalytic activity of MnFe2O4. For example, MnFe2O4 nanorods (MnFe2O4-NR) had a higher catalytic activity than MnFe2O4 nanospheres (MnFe2O4-NS) and MnFe2O4 nanocubes (MnFe2O4-NC) in OFX removal and H2O2 decomposition. Notably, the catalytic activity was remarkably enhanced with increasing the relative amount of Mn3+ and Fe2+ species on the surface. Based on the results from quenching experiments and quantitative determination of •OH radicals, a possible catalytic mechanism of MnFe2O4 was proposed. In addition, the stability and reusability of MnFe2O4-NR was ascertained, as the results suggested that MnFe2O4-NR was a stable and easily separated catalyst for heterogeneous Fenton process.










Similar content being viewed by others
Data availability
Not applicable.
References
Chen J, Wen WJ, Kong LJ, Tian SH, Ding FC, Xiong Y (2014) Magnetically separable and durable MnFe2O4 for efficient catalytic ozonation of organic pollutants. Ind Eng Chem Res 53:6297–6306
Chu XF, Jiang DL, Zheng CM (2007) The preparation and gas-sensing properties of NiFe2O4 nanocubes and nanorods. Sensors Actuators B Chem 123:793–797
Costa RCC, Lelis MFF, Oliveira LCA, Fabris JD, Ardisson JD, Rios RRVA, Silva CN, Lago RM (2006) Novel active heterogeneous Fenton system based on Fe3-xMxO4 (Fe, Co, Mn, Ni): the role of M2+ species on the reactivity towards H2O2 reactions. J Hazard Mater 129:171–178
Deng J, Feng SF, Ma XY, Tan CQ, Wang HY, Zhou SQ, Zhang TQ, Li J (2016) Heterogeneous degradation of Orange II with peroxymonosulfate activated by ordered mesoporous MnFe2O4. Sep Purif Technol 167:181–189
Ensing B, Buda F, Baerends EJ (2003) Fenton-like chemistry in water: oxidation catalysis by Fe(III) and H2O2. J Phys Chem 107:5722–5731
Fayazi M, Taher MA, Afzali D, Mostafavi A (2016) Enhanced Fenton-like degradation of methylene blue by magnetically activated carbon/hydrogen peroxide with hydroxylamine as Fenton enhancer. J Mol Liq 216:781–787
Fayazi M, Ghanei-Motlagh M (2020) Electrochemical mineralization of methylene blue dye using electro-Fenton oxidation catalyzed by a novel sepiolite/pyrite nanocomposite. Int J Environ Sci Technol 17:4541–4548
Feng JY, Hu XJ, Yue PL (2004) Discoloration and mineralization of orange II using different heterogeneous catalysts containing Fe: a comparative study. Environ Sci Technol 38:5773–5778
Fu HC, Ma SL, Zhao P, Xu SJ, Zhan SH (2019) Activation of peroxymonosulfate by graphitized hierarchical porous biochar and MnFe2O4 magnetic nanoarchitecture for organic pollutants degradation: sstructure dependence and mechanism. Chem Eng J 360:157–170
Gan GQ, Liu J, Zhu ZX, Yang Z, Zhang CL, Hou XH (2017) A novel magnetic nanoscaled Fe3O4/CeO2 composite prepared by oxidation-precipitation process and its application for degradation of orange G in aqueous solution as Fenton-like heterogeneous catalyst. Chemosphere 168:254–263
Gu ZJ, Xiang X, Fan GL, Li F (2008) Facile Synthesis and characterization of cobalt ferrite nanocrystals via a simple reduction-oxidation route. J Phys Chem C 112:18459–18466
Guo J, Al-Dahhan M (2003) Catalytic wet oxidation of phenol by hydrogen peroxide over pillared clay catalyst. Ind Eng Chem Res 42:2450–2460
Guo PZ, Zhang GL, Yu JQ, Li HL, Zhao XS (2012) Controlled synthesis, magnetic and photocatalytic properties of hollow spheres and colloidal nanocrystal clusters of manganese ferrite. Colloid Surface A: Physicochem Eng Aspects 395:168–174
Herney-Ramirez J, Vicente MA, Madeira LM (2010) Heterogeneous photo-Fenton oxidation with pillared clay-based catalysts for wastewater treatment: a review. Appl Catal B Environ 98:10–26
Hou XY, Feng J, Ren YM, Fan ZJ, Zhang ML (2010a) Synthesis and adsorption properties of spongelike porous MnFe2O4. Colloid Surface A: Physicochem Eng Aspects 363:1–7
Hou XY, Feng J, Wang ZQ, Liu XH, Zhang ML (2010b) Synthesis of MnFe2O4 nanofibres by hydrothermal method. J Funct Mater 41:1706–1708
Jiang F, Du XM, Zhao SH, Guo JX, Huang BJ, Huang X, Su QM, Zhang J, Du GH (2015) Preparation of carbon-coated MnFe2O4 nanospheres as high-performance anode materials for lithium-ion batteries. J Nanopart Res 17:173–181
Jin H, Tian XK, Nie YL, Zhou ZX, Yang C, Li Y, Lu LQ (2017) Oxygen vacancy promoted heterogeneous Fenton-like degradation of ofloxacin at pH 3.2-9.0 by Cu substituted magnetic Fe3O4@FeOOH nanocomposite. Environ Sci Technol 51:12699–12706
Li B, Zhu J (2014) Removal of p-chloronitrobenzene from groundwater: effectiveness and degradation mechanism of a heterogeneous nanoparticulate zero-valent iron (NZVI)-induced Fenton process. Chem Eng J 255:225–232
Li KY, Zhao YQ, Song CS, Guo XW (2017) Magnetic ordered mesoporous Fe3O4/CeO2 composites with synergy of adsorption and Fenton catalysis. Appl Surf Sci 45:526–534
Liang XL, Zhong YH, He HP, Yuan P, Zhu JX, Zhu SY, Jiang Z (2012) The application of chromium substituted magnetite as heterogeneous Fenton catalyst for the degradation of aqueous cationic and anionic dyes. Chem Eng J 191:177–184
Lin SS, Gurol MD (1998) Catalytic decomposition of hydrogen peroxide on iron oxide: kinetics, mechanism, and implications. Environ Sci Technol 32:1417–1423
Lindsey ME, Tarr MA (2000) Quantitation of hydroxyl radical during Fenton oxidation following a single addition of iron and peroxide. Chemosphere 41:409–417
Magalhaes F, Pereira MC, Botrel SEC, Fabris JD, Macedo WA, Mendonca R, Lago RM, Oliveira LCA (2007) Cr-containing magnetites Fe3-xCrxO4: the role of Cr3+ and Fe2+ on the stability and reactivity towards H2O2 reactions. Appl Catal A Gen 332:115–123
Melero JA, Calleja G, Martínez F, Molina R, Pariente MI (2007) Nanocomposite Fe2O3/SBA-15: an efficient and stable catalyst for the catalytic wet peroxidation of phenolic aqueous solutions. Chem Eng J 131:245–256
Messele SA, Soares OSGP, Órfão JJM, Stüber F, Bengoa C, Fortuny A, Fabregat A, Font J (2014) Zero-valent iron supported on nitrogen-containing activated carbon for catalytic wet peroxide oxidation of phenol. Appl Catal B Environ 154-155:329–338
Navalon S, Alvaro M, Garcia H (2010) Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Appl Catal B Environ 99:1–26
Nguyen TD, Phan NH, Do MH, Ngo KT (2011) Magnetic Fe2MO4 (M: Fe, Mn) activated carbons: fabrication, characterization and heterogeneous Fenton oxidation of methyl orange. J Hazard Mater 185:653–661
Orge CA, Orfao JJM, Pereira MFR, de Farias AMD, Neto RCR, Fraga MA (2011) Ozonation of model organic compounds catalysed by nanostructured cerium oxides. Appl Catal B Environ 103:190–199
Qin HD, Xiao R, Chen J (2018a) Catalytic wet peroxide oxidation of benzoic acid over Fe/AC catalysts: effect of nitrogen and sulfur co-doped activated carbon. Sci Total Environ 626:1414–1420
Qin HD, Xiao R, Shi W, Wang Y, Li H, Guo L, Cheng H, Chen J (2018b) Magnetic core-shell-structured Fe3O4@CeO2 as an efficient catalyst for catalytic wet peroxide oxidation of benzoic acid. RSC Adv 8:33972–33979
Qin HD, Cheng H, Li H, Wang Y (2020) Degradation of ofloxacin, amoxicillin and tetracycline antibiotics using magnetic core–shell MnFe2O4@C-NH2 as a heterogeneous Fenton catalyst. Chem Eng J 396:125304
Rey A, Faraldos M, Casas JA, Zazo JA, Bahamonde A, Rodríguez JJ (2009) Catalytic wet peroxide oxidation of phenol over Fe/AC catalysts: influence of iron precursor and activated carbon surface. Appl Catal B Environ 86:69–77
Tan C, Gao N, Fu D, Deng J, Deng L (2017) Efficient degradation of paracetamol with nanoscaled magnetic CoFe2O4 and MnFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Sep Purif Technol 175:47–57
Valdes-Solis T, Valle-Vigon P, Alvarez S, Marban G, Fuertes AB (2007) Manganese ferrite nanoparticles synthesized through a nanocasting route as a highly active Fenton catalyst. Catal Commun 8:2037–2042
Vignesh V, Subramanib K, Sathish M, Navamathavan R (2018) Electrochemical investigation of manganese ferrites prepared via a facile synthesis route for supercapacitor applications. Colloid Surface A: Physicochem Eng Aspects 538:668–677
Wan Z, Wang JL (2017) Degradation of sulfamethazine antibiotics using Fe3O4-Mn3O4 nanocomposite as a Fenton-like catalyst. J Chem Technol Biotechnol 92:874–883
Wang J, Chen QW, Hou BY, Peng ZM (2004) Synthesis and magnetic properties of single-crystals of MnFe2O4 nanorods. Eur J Inorg Chem 6:1165–1168
Wang J, Liu C, Qi J, Li J, Sun X, Shen J, Han W, Wang L (2018) Enhanced heterogeneous Fenton-like systems based on highly dispersed Fe0-Fe2O3 nanoparticles embedded ordered mesoporous carbon composite catalyst. Environ Pollut 243:1068–1077
Wang YB, Zhao HY, Li MF, Fan JQ, Zhao GH (2014) Magnetic ordered mesoporous copper ferrite as a heterogeneous Fenton catalyst for the degradation of imidacloprid. Appl Catal B Environ 147:534–545
Xia M, Long MC, Yang YD, Chen C, Cai WM, Zhou BX (2011) A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution. Appl Catal B Environ 110:118–125
Xu L, Wang J (2012) Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ Sci Technol 46:10145–10153
Zazo JA, Casas JA, Mohedano AF, Rodríguez JJ (2006) Catalytic wet peroxide oxidation of phenol with a Fe/active carbon catalyst. Appl Catal B Environ 65:261–268
Zha SX, Cheng Y, Gao Y, Chen ZL, Megharaj M, Naidu R (2014) Nanoscale zero-valent iron as a catalyst for heterogeneous Fenton oxidation of amoxicillin. Chem Eng J 255:141–148
Zhang GK, Gao YY, Zhang AL, Guo YD (2010) Fe2O3-pillared rectorite as an efficient and stable Fenton-Like heterogeneous catalyst for photodegradation of organic contaminants. Environ Sci Technol 44:6384–6389
Zhang H, Liu JG, Ou CJ, Faheem, Shen JY, Yu HX, Jiao ZH, Han WQ, Sun XY, Li JS, Wang LJ (2017) Reuse of Fenton sludge as an iron source for NiFe2O4 synthesis and its application in the Fenton-based process. J Environ Sci 53:1–8
Zhang Z, Wang Y, Tan Q, Zhong Z, Su F (2013) Facile solvothermal synthesis of mesoporous manganese ferrite (MnFe2O4) microspheres as anode materials for lithium-ion batteries. J Colloid Interface Sci 398:185–192
Zhao H, Dong YM, Wang GL, Jiang PP, Zhang JJ, Wu LN, Li K (2013) Novel magnetically separable nanomaterials for heterogeneous catalytic ozonation of phenol pollutant: NiFe2O4 and their performances. Chem Eng J 219:295–302
Zhen L, He K, Xu CY, Shao WZ (2008) Synthesis and characterization of single crystalline MnFe2O4 nanorods via a surfactant-free hydrothermal route. J Magn Magn Mater 320:2672–2675
Zheng CM, Yang CW, Cheng XZ, Xu SC, Fan ZP, Wang GH, Wang SB, Guan XF, Sun XH (2017) Specifically enhancement of heterogeneous Fenton-like degradation activities for ofloxacin with synergetic effects of bimetallic Fe-Cu on ordered mesoporous silicon. Sep Purif Technol 189:357–365
Materials availability
Not applicable.
Funding
This study was financially supported by the Department of Education of Guizhou Province (QJHKYZ [2020] 042), National Natural Science Foundation of China (21805211, 51862033), and Science and Technology Foundation of Guizhou Province (JC[2017]1185, JC[2018]1164, ZC[2020]2Y037, PTRC[2017]5604).
Author information
Authors and Affiliations
Contributions
Hangdao Qin conducted experiments and analyzed experimental data, was a major contributor in writing the original manuscript. Yingchang Yang interpreted the experimental data, and also a major contributor in writing the original manuscript. Wei Shi and Yuanbin She wrote, reviewed, and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo Torres-Palma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, H., Yang, Y., Shi, W. et al. Heterogeneous Fenton degradation of ofloxacin catalyzed by magnetic nanostructured MnFe2O4 with different morphologies. Environ Sci Pollut Res 28, 26558–26570 (2021). https://doi.org/10.1007/s11356-021-12548-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12548-y