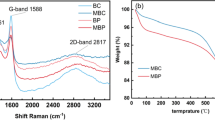
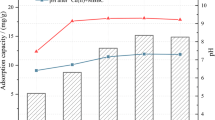
Abstract
Biochar (BC) has been widely used to remove heavy metals from wastewater. However, due to the hydrophobicity of BC and the lack of its surface functional groups, the effect of metal ions adsorption onto BC is limited. In order to improve the adsorption efficiency, l-cysteine was used to modify biochar derived from pomelo peel (PP) to regulate surface structure. The characteristics of BC and cysteine/biochar composite (cys/BC) were analyzed by various characterization methods. Results showed that the hydrophilicity of biochar was enhanced, and the number of surface functional groups was increased, resulting to strong adsorption ability of Ag(I) (618.9 mg/g), Pb(II) (274.5 mg/g), and As(V) (34.7 mg/g) for cys/BC, which increased approximately by 15%, 35%, and 29% compared with that of BC, respectively. The adsorption process of Pb(II) onto cys/BC was fitted better by the Freundlich isotherm model and for Ag(I) and As(V) by the Langmuir isotherm model. Moreover, the adsorption kinetics followed pseudo-second-order equation and the adsorption process was controlled by the intraparticle diffusion for Ag(I), Pb(II), and As(V) adsorption onto cys/BC. In addition, the adsorption capacities of cys/BC for Ag(I), Pb(II), and As(V) decreased slightly after five adsorption/desorption cycles. Finally, the multiple adsorption mechanisms including functional groups, pore adsorption, surface complexation, and cations-π were analyzed. The paper demonstrated that the cys/BC composite could be reused as effective adsorbents for removing contaminants in the environment.






Similar content being viewed by others
References
Aguado J, Arsuaga JM, Arencibia A, Lindo M, Gascón V (2009) Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J Hazard Mater 163:213–221
Antochshuk V, Jaroniec M (2002) 1-Allyl-3-propylthiourea modified mesoporous silica for mercury removal. Chem Commun 3:258–259
Banazadeh A, Mozaffari S, Osolib B (2015) Facile synthesis of cysteine functionalized magnetic graphene oxide nanosheets: application in solid phase extraction of cadmium from environmental sample. J Environ Chem Eng 3:2801–2808
Celik Z, Gülfen M, Aydın AO (2010) Synthesis of a novel dithiooxamide–formaldehyde resin and its application to the adsorption and separation of silver ions. J Hazard Mater 174:556–562
Chen XC, Chen GC, Chen LG, Chen YX, Lehmann J, McBride MB, Hay AG (2011) Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour Technol 102:8877–8884
Chen T, Zhang YX, Wang HT, Lu WJ, Zhou ZY, Zhang YC, Ren LL (2014) Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour Technol 164:47–54
Chen Y, Shi J, Du Q, Zhang H, Cui Y (2019) Antibiotic removal by agricultural waste biochars with different forms of iron oxide. RSC Adv 9(25):14143–14153
Dai Y, Zhang N, Xing C, Cui Q, Sun Q (2019) The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: a review. Chemosphere 223:12–27
Disbudak A, Bektas S, Patir S, Genc O, Denizli A (2002) Cysteine-metal affinity chromatography: determination of heavy metal adsorption properties. Sep Purif Technol 26:273–281
Fan JJ, Cai C, Chi H, Reid BJ, Coulon F, Zhang Y, Hou Y (2020) Remediation of cadmium and lead polluted soil using thiol-modified biochar. J Hazard Mater 388:122037–122048
Feng Y, Liu P, Wang YX, Finforck YZ, Xie XJ, Su CL, Liu N, Yang YY, Xu Y (2020) Distribution and speciation of iron in Fe-modified biochars and its application in removal of As(V), As(III), Cr(VI), and Hg(II): An X-ray absorption study. J Hazard Mater 384:121342–121352
Fuertes AB, Camps Arbestain M, Sevilla M, Maciá-Agulló JA, Fiol S, López R, Smernik RJ, Aitkenhead WP, Arce F, Macias F (2010) Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Aust J Soil Res 48:618–626
Georgakilas V, Otyepka M, Bourlinos AB, Chandra V, Kim N, Kemp KC, Hobza P, Zboril R, Kim KS (2012) Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev 112:6156–6214
Haghseresht F, Wang S, Do DD (2009) A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Appl Clay Sci 46:369–375
Harvey OR, Herbert BE, Rhue RD, Kuo LJ (2011) Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ Sci Technol 45:5550–5556
He Q (1991) Study on synthesis and sorption properties of spherical thiourea polycondensate-type resins for silver cation. Ion Exch Adsorp 7:42–46
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng 70:115–124
Hu X, Ding ZH, Zimmerman AR, Wang S, Gao B (2015) Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res 68:206–216
Jeguirim M, Limousy L, Dutournie P (2014) Pyrolysis kinetics and physicochemical properties of agropellets produced from spent ground coffee blended with conventional biomass. Chem Eng Res Des 92:1876–1882
Jung K, Ok YS, Chang SX (2011) Sulfate adsorption properties of acid-sensitive soils in the Athabasca oil sands region in Alberta, Canada. Chemosphere 84:457–463
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253
Kolodynska D, Krukowska J, Thomas P (2017) Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem Eng 307:353–363
Kwapinski W, Byrne CMP, Kryachko E, Wolfram P, Adley C, Leahy JJ, Novotny EH, Hayes MHB (2010) Biochar from biomass and waste. Waste Biomass Valorizat 1:177–189
Lagergren S (1898) Zur theorie der sogenannten adsorption gelosterstoffe, Kungliga Svenska Vetenskapsakademiens, Handlingar Band 24: 1–39
Li X, Zhou H, Wu W, Wei S, Xu Y, Kuang Y (2015) Studies of heavy metal ion adsorption on Chitosan/Sulfydryl-functionalized graphene oxide composites. J Colloid Interface Sci 448:389–397
Li BQ, Yuan WH, Li L (2016) Adsorption of Pb2+ and Cd2+ on graphene nanosheets prepared using thermal exfoliation. Acta Phys -Chim Sin 32:997–1004
Liang Q, Ye L, Huang ZH, Xu Q, Bai Y, Kang F, Yang QH (2014) A honeycomb-like porous carbon derived from pomelo peel for use in high-performance supercapacitors. Nanoscale 6(22):13831–13837
Lin LN, Huang Q, Liu ZQ, Song ZG (2017) Preparation of biochar-ferro manganese oxide composite material and properties of removal of arsenic(III) from aqueous solution. J Agric Res Environ 34:182–188
Liu Y, Li Y, Yan XP (2008) Preparation, characterization, and application of l-cysteine functionalized multiwalled carbon nanotubes as a selective sorbent for separation and preconcentration of heavy metals. Adv Funct Mater 18:1536–1543
Liu LQ, Huang YJ, Zhang SP, Gong Y, Su YH, Cao JH, Hu HJ (2019) Adsorption characteristics and mechanism of Pb(II) by agricultural waste-derived biochars produced from a pilot-scale pyrolysis system. Waste Manag 100:287–295
Lu Y, He J, Luo G (2013) An improved synthesis of chitosan bead for Pb(II) adsorption. Chem Eng J 226:271–278
Luo MK, Lin H, He YH, Zhang Y (2020) The influence of corncob-based biochar on remediation of arsenic and cadmium in yellow soil and cinnamon soil. Sci Total Environ 717:137014–137022
Mudhoo A, Sharma SK, Garg VK, Tseng CH (2011) Arsenic: an overview of applications, health, and environmental concerns and removal processes. Environ Sci Technol 41:435–519
Reddad Z, Gerente C, Andres Y, Le Cloirec P (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36:2067–2073
Shao J, Yang YM, Shi CG (2010) Preparation and adsorption properties for metal ions of chitin modified by l-cysteine. J Appl Polym Sci 88:2575–2579
Shen Z, Jin F, Wang F, McMillan O, Al-Tabbaa A (2015) Sorption of lead by Salisbury biochar produced from British broadleaf hardwood. Bioresour Technol 193:553–556
Silva LFO, Sampaio CH, Guedes A, de Vallejuelo SFO, Madariaga JM (2012) Multianalytical approaches to the characterisation of minerals associated with coals and the diagnosis of their potential risk by using combined instrumental microspectroscopic techniques and thermodynamic speciation. Fuel 94:52–63
Song M, Wei Y, Cai S, Yu L, Zhong Z, Jin B (2018) Study on adsorption properties and mechanism of Pb. Sci Total Environ 618:1416–1422
Stathatos E, Lianos P, Falaras P, Siokou A (2000) Photocatalytically deposited silver nanoparticles on mesoporous TiO2 films. Langmuir 16:2398–2400
Taghavi M, Zazouli MA, Yousefi Z, Akbari-adergani B (2015) Kinetic and isotherm modeling of Cd (II) adsorption by l-cysteine functionalized multi-walled carbon nanotubes as adsorbent. Environ Monit Assess 187:682–691
Trakal L, Veselská V, Šafařík I, Vítková M, Číhalová S, Komárek M (2016) Lead and cadmium sorption mechanisms on magnetically modified biochars. Bioresour Technol 203:318–324
Uzun L, Türkmen D, Yılmaz E, Bektas S, Denizli A (2008) Cysteine functionalized poly (hydroxyethyl methacrylate) monolith for heavy metal removal. Colloids and Surfaces A: Physicochem Eng Aspects 330:161–167
Wang XL, Xing BS (2007) Sorption of organic contaminants by biopolymerderived chars. Environ Sci Technol 41:8342–8348
Wang J, Chen GH, Chen J (2009) Investigation on aqueous Hg(II) adsorption properties by thiol-modified activated carbon. J Environ Eng 3:219–222
Wang HY, Gao B, Wang SS, Fang J, Xue YW, Yang K (2015) Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour Technol 197:356–362
Wang QH, Lai ZY, Jun M, Chu DM, Zang XR (2020) Converting industrial waste cork to biochar as Cu (II) adsorbent via slow pyrolysis. Waste Manag 105:102–109
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–60
Wei Z, Seo Y (2010) Trichloroethylene (TCE) adsorption using sustainable organic mulch. J Hazard Mater 181:147–153
Xiang L, Zeng LJ, Du PP, Wang XD, Wu XL, Sarkar B, Lü HX, Li YW, Li H, Mo CH, Wang HL, Cai QY (2020) Effects of rice straw biochar on sorption and desorption of di-n-butyl phthalate in different soil particle-size fractions. Sci Total Environ 702:134878–134891
Yan PF, Ye MY, Sun SY, Xiao X, Dai WC, Zhang N (2016) Removal performances and mechanisms of action towards ethylenediaminetetraacetic acid nickel (II) salt by dithiocarbamate compounds having different carbon chain lengths. J Clean Prod 122:308–314
Yang Y, Shao J (2000) Synthesis of sulfhydryl chitin and its adsorption properties for heavy metal ions. J Appl Polym Sci 77:151–155
Yao Y, Gao B, Wu F, Zhang CZ, Yang LY (2015) Engineered biochar from biofuel residue: characterization and its silver removal potential. Appl Mater Interfaces 7:10634–10640
Zhang M, Gao B (2013) Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem Eng J 226:286–292
Zheng W, Li XM, Wang F, Yang Q, Deng P, Zeng GM (2008) Adsorption removal of cadmium and copper from aqueous solution by areca a food waste. J Hazard Mater 157:490–495
Zhou YM, Gao B, Zimmerman AR, Fang J, Sun YN, Cao XD (2013) Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem Eng J 231:512–518
Zhou YM, Gao B, Zimmerman AR, Cao X (2014a) Biochar-supported zerovalent iron reclaims silver from aqueous solution to form antimicrobial nanocomposite. Chemosphere 117:801–805
Zhou YM, Gao B, Zimmerman AR, Cao XD (2014b) Biochar-supported zero-valent iron for removal of various contaminants from aqueous solutions. Bioresour Technol 152:538–542
Zhou YM, Wang XH, Zhang M, Jin Q, Gao B, Ma TS (2014c) Removal of Pb (II) and malachite green from aqueous solution by modified cellulose. Cellulose 21:2797–2809
Zhou Z, Liu YG, Liu SB, Liu HY, Zeng GM, Tan XF (2017) Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chem Eng J 314:223–231
Zuo WQ, Chen C, Cui HJ, Fu ML (2017) Enhanced removal of Cd (II) from aqueous solution using CaCO3 nanoparticle modified sewage sludge biochar. RSC Adv 7:16238–16243
Funding
This work was supported by Science and Technology Development Special Fund of Guangdong Province (2017A070712013) and the Analytical and Testing Center of SCNU of TEM measurement (No. 16 KJ20).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 310 kb)
Rights and permissions
About this article
Cite this article
Li, B., Gong, J., Fang, J. et al. Cysteine chemical modification for surface regulation of biochar and its application for polymetallic adsorption from aqueous solutions. Environ Sci Pollut Res 28, 1061–1071 (2021). https://doi.org/10.1007/s11356-020-10558-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10558-w