Abstract
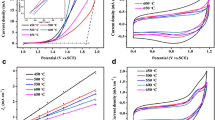
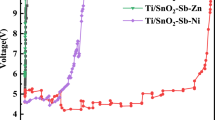
Titanium-based SnO2 with Sb dopant (Ti/Sb-SnO2) was of interest in the field of electro-catalytic oxidation due to its high organic oxidation rates. However, the relatively poor mass transfer performance and short service time limited its practical application. To overcome this problem, Ti/Sb-SnO2 electrode was fabricated on mesh substrate and used as the anode for electrochemical oxidization of phenol. Compared to the anode prepared on planar Ti, the mesh anode with compact and uniform catalyst surface lowered electron transfer resistance and higher Oads content (17.41%), which benefited the generation of hydroxyl radicals (·OH) (increment of 24.5%). In addition, this structure accelerated the fluid perturbation around electrode in microscopic scale as the COMSOL simulation result indicated; the electric potential on mesh anode varied regularly along the undulant terrain of electrode so that the mass transfer coefficient was enhanced by 1.67 times. These structure-dependent characteristics contributed to the superior electro-catalytic performance toward degradation of phenol. Experimental results showed that mesh anode had a higher TOC removal efficiency of 90.6% and mineralization current efficiency of 20.1% at current density of 10 mA cm−2, which was 9.95% and 21.6% higher than the planar anode, and the service lifetime was 1.89 times longer than planar anode. This highly electro-catalytically active and stable Ti/Sb-SnO2 mesh electrode showed a potential application prospect toward electro-catalytic degradation process.






Similar content being viewed by others
References
Adams B, Tian M, Chen A (2009) Design and electrochemical study of SnO2-based mixed oxide electrodes, Electrochim Acta (54):1491-1498
Balci B O, Nihal; Cherrier, Richard; Oturan, Mehmet A. (2009) Degradation of atrazine in aqueous medium by electrocatalytically generated hydroxyl radicals. A kinetic and mechanistic study, Water Res (43):1924–1934
Beer HB (1972) Electrodes and coating thereof. In: US
Brown C J, Pletcher D, Walsh F C, Hammond J K, Robinson D (1992) Local mass-transport effects in the Fmoi laboratory electrolyzer J Appl Electrochem (22):613-619
Canizares P, Lobato J, Paz R, Rodrigo M A, Saez C (2007) Advanced oxidation processes for the treatment of olive-oil mills wastewater, Chemosphere (67):832-838
Cao J, Zhao H, Cao F, Zhang J, Cao C (2009) Electrocatalytic degradation of 4-chlorophenol on F-doped PbO2 anodes, Electrochim Acta (54):2595-2602
Chaiyont R, Badoe C, de Leon C P, Nava J L, Recio F J, Sires I, Herrasti P, Walsh F C (2013) Decolorization of methyl orange dye at IrO2-SnO2-Sb2O5 coated titanium anodes, Chem Eng Technol (36):123-129
Chen A C, Nigro S (2003) Influence of a nanoscale gold thin layer on Ti/SnO2-Sb2O5 electrodes, J Phys Chem B (107):13341-13348
Chen Y, Hong L, Xue H M, Han W Q, Wang L J, Sun X Y, Li J S (2010) Preparation and characterization of TiO2-NTs/SnO2-Sb electrodes by electrodeposition, J Electroanal Chem (648):119-127
Chen Y, Li H, Liu W, Tu Y, Zhang Y, Han W, Wang L (2014) Electrochemical degradation of nitrobenzene by anodic oxidation on the constructed TiO2-NTs/SnO2-Sb/PbO2 electrode, Chemosphere (113):48-55
Chu D, Xu M, Lu J, Zheng P, Qin G, Yuan X (2008) Electrocatalytic reduction of diethyl oximinomalonate at a Ti/nanoporous TiO2 electrode, Electrochem Commun (10):350-353
Comninellis C (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste-water treatment, Electrochim Acta (39):1857-1862
Comninellis C (1992) Electrochemical treatment of waste-water containing phenol, Process Saf Environ(70):219-224
Comninellis C, Pulgarin C (1993) Electrochemical oxidation of phenol for wastewater treatment using SnO2, anodes, J Appl Electrochem. (23):108-112
Comninellis C V, G. P. (1991) Characterization of DSA-type oxygen evolving electrodes-choice of a coating, J Appl Electrochem (21):335–345
CorreaLozano B, Comninellis C, DeBattisti A (1996) Physicochemical properties of SnO2-Sb2O5 films prepared by the spray pyrolysis technique, J Electrochem Soc (143):203-209
Cruz-Diaz M R, Rivero E P, Rodriguez F A, Dominguez-Bautista R (2018) Experimental study and mathematical modeling of the electrochemical degradation of dyeing wastewaters in presence of chloride ion with dimensional stable anodes (DSA) of expanded meshes in a FM01-LC reactor, Electrochim Acta (260):726-737
Cui Y H, Feng Y J, Li X Y (2011) Kinetics and efficiency analysis of electrochemical oxidation of phenol: influence of anode materials and operational conditions, Chem Eng Technol (34):265-272
Cui Y H, Feng Y J, Liu Z Q (2009a) Influence of rare earths doping on the structure and electro-catalytic performance of Ti/Sb-SnO2 electrodes, Electrochim Acta (54):4903-4909
Cui Y H, Li X Y, Chen G H (2009b) Electrochemical degradation of bisphenol a on different anodes, Water Res (43):1968-1976
Ding H Y, Feng Y J, Lue J W, Liu J F (2007) Detection of hydroxyl radicals during electrolysis of Ti/SnO2 electrode and analysis of the electrocatalytic mechanism, Chinese J Anal Chem (35):1395-1399
Duan Y, Chen Y, Wen Q, Duan T G (2016) Fabrication of dense spherical and rhombic Ti/Sb-SnO2 electrodes with enhanced electrochemical activity by colloidal electrodeposition, J Electroanal Chem (768):81-88
Feng Y, Cui Y H, Liu J, Logan B E (2010) Factors affecting the electro-catalytic characteristics of Eu doped SnO2/Sb electrode J Hazard Mater (178):29-34
Feng Y J, Cui Y H, Logan B, Liu Z Q (2008) Performance of Gd-doped Ti-based Sb-SnO2 anodes for electrochemical destruction of phenol Chemosphere (70):1629-1636
Griffiths M, de Leon C P, Walsh F C (2005) Mass transport in the rectangular channel of a filter-press electrolyzer (the FM01-LC reactor), Aiche J (51):682-687
Guinea E, Arias C, Cabot P L, Garrido J A, Rodriguez R M, Centellas F, Brillas E (2008) Mineralization of salicylic acid in acidic aqueous medium by electrochemical advanced oxidation processes using platinum and boron-doped diamond as anode and cathodically generated hydrogen peroxide, Water Res (42):499-511
Huang LL, Li D, Liu JF, Yang LS, Dai CC, Ren NQ, Feng YJ (2020) Construction of TiO2 nanotube clusters on Ti mesh for immobilizing Sb-SnO2 to boost electrocatalytic phenol degradation. J Hazard Mater 393:122329
Kapalka A, Foti G, Comninellis C (2008) Kinetic modelling of the electrochemical mineralization of organic pollutants for wastewater treatment, J Appl Electrochem (38):7-16
Li G Z, Li G, Wang H, Xiang C S, Zhuang J D, Liu Q, Tang H P (2015) Preparation of Sb doped Nano SnO2/porous Ti electrode and its degradation of methylene orange, Rare Metal Mater Eng (44):1326-1330
Li X Y, Cui Y H, Feng Y J, Xie Z M, Gu J D (2005) Reaction pathways and mechanisms of the electrochemical degradation of phenol on different electrodes, Water Res (39):1972-1981
Lund H B M, Organic chemistry, Marcel Dekker, New York, 1991
Peng X S, Koczkur K, Nigro S, Chen A C (2004) Fabrication and electrochemical properties of novel nanoporous platinum network electrodes Chem Commun2872-2873
Samet Y, Agengui L, Abdelhédi R (2010) Electrochemical degradation of chlorpyrifos pesticide in aqueous solutions by anodic oxidation at boron-doped diamond electrodes, Chem Eng J (161):167-172
Shao D, Li X L, Xu H, Yan W (2014a) An improved stable Ti/Sb-SnO2 electrode with high performance in electrochemical oxidation processes, RSC Adv (4):21230-21237
Shao D, Yan W, Cao L, Li X L, Xu H (2014b) High-performance Ti/Sb-SnO2/Pb3O4 electrodes for chlorine evolution: preparation and characteristics, J Hazard Mater (267):238-244
Sopaj F R, M. A. Oturan, N. Podvorica, F. I. Pinson, J. Oturan, M. A. (2015) Influence of the anode materials on the electrochemical oxidation efficiency. Application to oxidative degradation of the pharmaceutical amoxicillin, Chem Eng J (262):286–294
Stucki S, Kotz R, Carcer B, Suter W (1991) Electrochemical waste-water treatment using high overvoltage anodes .2. Anode performance and applications, J Appl Electrochem (21):99–104
Tran L H, Drogui P, Mercier G, Blais J F (2009) Electrolytic oxidation of Polynuclear aromatic hydrocarbons from creosote solution using Ti/IrO2 and Ti/SnO2 circular mesh electrodes, J Environ Eng-Asce (135):1051-1062
Wang H C, Cheng H Y, Cui D, Zhang B, Wang S S, Han J L, Su S G, Chen R, Wang A J (2017) Corrugated stainless-steel mesh as a simple engineerable electrode module in bio-electrochemical system: hydrodynamics and the effects on decolorization performance, J Hazard Mater (338):287-295
Wei J, Feng Y, Sun X, Liu J, Zhu L (2011a) Effectiveness and pathways of electrochemical degradation of pretilachlor herbicides, J Hazard Mater (189):84-91
Wei J Z, Feng Y J, Sun X J, Liu J F, Zhu L M (2011b) Effectiveness and pathways of electrochemical degradation of pretilachlor herbicides, J Hazard Mater (189):84-91
Xu A L, Dai X, Wei K J, Han W Q, Li J S, Sun X Y, Shen J Y, Wang L J (2017) Preparation and characterization of a TiO2-NT/SnO2-Sb tubular porous electrode with long service lifetime for wastewater treatment process, RSC Adv (7):37806-37814
Xu H Q, Li A P, Qi Q, Jiang W, Sun Y M (2012) Electrochemical degradation of phenol on the La and Ru doped Ti/SnO2-Sb electrodes, Korean J Chem Eng (29):1178-1186
Xu L, Guo Z, Du L S (2013) Anodic oxidation of azo dye CI acid red 73 by the yttrium-doped Ti/SnO2-Sb electrodes, Front Chem Sci Eng (7):338-346
Yang L S, Liu J F, Huang L L, Zhang Z H, Yu Y L, Liu J, Logan B E, Feng Y J (2018) Fabrication of Nano-structured stacked sphere SnO2-Sb electrode with enhanced performance using a situ Solvothermal synthesis method, J Electrochem Soc (165):E208-E213
Zhang H, Ran X N, Wu X G (2012) Electro-Fenton treatment of mature landfill leachate in a continuous flow reactor, J Hazard Mater (241):259-266
Zhang W, Robichaud M, Ghali E, Houlachi G (2016) Electrochemical behavior of mesh and plate oxide coated anodes during zinc electrowinning, T Nonferr Metal Soc (26):589-598
Zhao W, Xing J T, Chen D H, Jin D Y, Shen J (2016) Electrochemical degradation of musk ketone in aqueous solutions using a novel porous Ti/SnO2-Sb2O3/PbO2 electrodes, J Electroanal Chem (775):179-188
Acknowledgments
This research was supported by the National Key Research and Development Program (Grant No. 2017YFA0207204) and the International Cooperation Project between China and US (No. 21673061). The research also got the support from the Natural Science Foundation of China (No. 21972036) and from State Key Lab of Urban Water Resource & Environment (2018DX01). The authors also thank to the support from the Innovation Team in Key Areas, Ministry of Science and Technology of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philipp Gariguess
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 2419 kb)
Rights and permissions
About this article
Cite this article
Huang, L., Li, D., Liu, J. et al. Enhanced mass transfer and service time of mesh Ti/Sb-SnO2 electrode for electro-catalytic oxidation of phenol. Environ Sci Pollut Res 27, 42072–42081 (2020). https://doi.org/10.1007/s11356-020-10070-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10070-1