Abstract
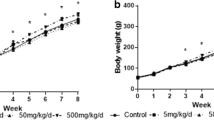
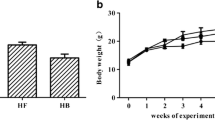
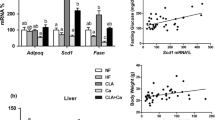
Di (2-ethylhexyl) phthalate (DEHP) and high-fat diet (HFD) could induce lipid metabolic disorder. This study was undertaken to identify the effect of DNA methylation of JAK3/STAT5/PPARγ on lipid metabolic disorder induced by DEHP and HFD. Wistar rats were divided into a normal diet (ND) group and HFD group. Each diet group treated with DEHP (0, 5, 50, 500 mg/kg/d) for 8 weeks’ gavage. The DNA-methylated levels of PPARγ, JAK3, STAT5a, and STAT5b in rats’ livers and adipose were analyzed with MethylTarget. The lipid levels of rats’ livers and adipose were detected with ELISA. Results showed in ND group that the DNA methylation levels of PPARγ, JAK3 in livers, and STAT5b in adipose were lower in 500 mg/kg/d group than the control. And the level of total cholesterol (TC) in adipose was higher in 500 mg/kg/d group than the control. In HFD group, the DNA methylation level of JAK3 was the lowest in livers and the highest in adipose in 50 mg/kg/d group. And the level of TC in livers was the lowest in 50 mg/kg/d group. In the 500 mg/kg/d group, the DNA methylation level of STAT5b was lower in livers and higher in adipose in HFD group than that in ND group. And the levels of TC in livers were lower in HFD group than those in ND group. Therefore, DNA methylation of JAK3/STAT5/PPARγ regulated the changes in lipid levels induced by DEHP and HFD in adolescent rats.





Similar content being viewed by others
References
Amara I, Timoumi R, Annabi E, Neffati F, Najjar MF, Bouaziz C, Abid-Essefi S (2019) Di (2-ethylhexyl) phthalate induces cardiac disorders in balb/c mice. Environ Sci Pollut Res Int 26:7540–7549
Bastos Sales L, van Esterik JCJ, Hodemaekers HM, Lamoree MH, Hamers T, van der Ven LTM, Legler J (2018) Analysis of lipid metabolism, immune function, and neurobehavior in adult c57bl/6jxfvb mice after developmental exposure to di (2-ethylhexyl) phthalate. Front Endocrinol 9:684
Chang CC, Sia KC, Chang JF, Lin CM, Yang CM, Huang KY, Lin WN (2019) Lipopolysaccharide promoted proliferation and adipogenesis of preadipocytes through jak/stat and ampk-regulated cpla2 expression. Int J Med Sci 16:167–179
Chen Z, Yu Y, Cai J, Li H (2019) Emerging molecular targets for treatment of nonalcoholic fatty liver disease. Trends Endocrinol Metab 30:903–914
Collaboration NCDRF (2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387:1377–1396
Dale CE, Fatemifar G, Palmer TM, White J, Prieto-Merino D, Zabaneh D, Engmann JEL, Shah T, Wong A, Warren HR, McLachlan S, Trompet S, Moldovan M, Morris RW, Sofat R, Kumari M, Hyppönen E, Jefferis BJ, Gaunt TR, Ben-Shlomo Y, Zhou A, Gentry-Maharaj A, Ryan A, UCLEB Consortium; METASTROKE Consortium, Mutsert R, Noordam R, Caulfield MJ, Jukema JW, Worrall BB, Munroe PB, Menon U, Power C, Kuh D, Lawlor DA, Humphries SE, Mook-Kanamori DO, Sattar N, Kivimaki M, Price JF, Davey Smith G, Dudbridge F, Hingorani AD, Holmes MV, Casas JP (2017) Causal associations of adiposity and body fat distribution with coronary heart disease, stroke subtypes, and type 2 diabetes mellitus: a mendelian randomization analysis. Circulation 135:2373–2388
de Moura BH, Amaral D, do Nascimento JN, Machado DC, de Sousa Araujo TA, de Albuquerque UP et al (2018) Spondias tuberosa inner bark extract exert antidiabetic effects in streptozotocin-induced diabetic rats. J Ethnopharmacol 227:248–257
Eldor R, DeFronzo RA, Abdul-Ghani M (2013) In vivo actions of peroxisome proliferator-activated receptors: glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care 36(Suppl 2):S162–S174
Ghaznavi H, Kiani AA, Soltanpour MS (2018) Association study between DNA methylation and genetic variation of apoe gene with the risk of coronary artery disease. Mol Biol Res Commun 7:173–179
Gomaa AA, Farghaly HSM, El-Sers DA, Farrag MM, Al-Zokeim NI (2018) Inhibition of adiposity and related metabolic disturbances by polyphenol-rich extract of boswellia serrata gum through alteration of adipo/cytokine profiles. Inflammopharmacology 27:549–559
Greenhill C (2015) Diabetes: DNA methylation affects t2dm risk. Nat Rev Endocrinol 11:505
Grimley PM, Dong F, Rui H (1999) Stat5a and stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev 10:131–157
Hernandez-Saavedra D, Stanford KI (2019) The regulation of lipokines by environmental factors. Nutrients 11:2422
Iqbal J, Walsh MT, Hammad SM, Hussain MM (2017) Sphingolipids and lipoproteins in health and metabolic disorders. Trends Endocrinol Metab 28:506–518
Jaruratanasirikul S, Thammaratchuchai S, Sriplung H (2017) Trends of childhood diabetes in southern Thailand: 20-year experience in a tertiary medical center. World J Pediatr: WJP 13:566–570
Jia Y, Liu T, Zhou L, Zhu J, Wu J, Sun D et al (2016) Effects of di-(2-ethylhexyl) phthalate on lipid metabolism by the jak/stat pathway in rats. Int J Environ Res Public Health 13:1085
Kim SH, On JW, Pyo H, Ko KS, Won JC, Yang J, Park MJ (2018) Percentage fractions of urinary di(2-ethylhexyl) phthalate metabolites: association with obesity and insulin resistance in Korean girls. PLoS One 13:e0208081
Lee JB, Yoon SJ, Lee SH, Lee MS, Jung H, Kim TD, Yoon SR, Choi I, Kim IS, Chung SW, Lee HG, Min JK, Park YJ (2017) Ginsenoside rg3 ameliorated hfd-induced hepatic steatosis through downregulation of stat5-ppargamma. J Endocrinol 235:223–235
Lu X, Huang J, Mo Z, He J, Wang L, Yang X, Tan A, Chen S, Chen J, Gu CC, Chen J, Li Y, Zhao L, Li H, Hao Y, Li J, Hixson JE, Li Y, Cheng M, Liu X, Cao J, Liu F, Huang C, Shen C, Shen J, Yu L, Xu L, Mu J, Wu X, Ji X, Guo D, Zhou Z, Yang Z, Wang R, Yang J, Yan W, Peng X, Gu D (2016) Genetic susceptibility to lipid levels and lipid change over time and risk of incident hyperlipidemia in Chinese populations. Circ Cardiovasc Genet 9:37–44
Maradonna F, Carnevali O (2018) Lipid metabolism alteration by endocrine disruptors in animal models: an overview. Front Endocrinol 9:654
Marginean CO, Marginean C, Melit LE (2018) New insights regarding genetic aspects of childhood obesity: a minireview. Front Pediatr 6:271
Matsumoto T, Kiuchi S, Murase T (2019) Synergistic activation of thermogenic adipocytes by a combination of ppar gamma activation, smad3 inhibition and adrenergic receptor activation ameliorates metabolic abnormalities in rodents. Diabetologia 62:1915–1927
Meirhaeghe A, Fajas L, Gouilleux F, Cottel D, Helbecque N, Auwerx J, Amouyel P (2003) A functional polymorphism in a stat5b site of the human ppar gamma 3 gene promoter affects height and lipid metabolism in a french population. Arterioscler Thromb Vasc Biol 23:289–294
Murtaza M, Khan G, Aftab MF, Afridi SK, Ghaffar S, Ahmed A, Hafizur RM, Waraich RS (2017) Cucurbitacin e reduces obesity and related metabolic dysfunction in mice by targeting jak-stat5 signaling pathway. PLoS One 12:e0178910
Ni HX, Yu NJ, Yang XH (2010) The study of ginsenoside on ppar gamma expression of mononuclear macrophage in type 2 diabetes. Mol Biol Rep 37:2975–2979
Nikolova-Karakashian M (2018) Alcoholic and non-alcoholic fatty liver disease: focus on ceramide. Adv Biol Regul 70:40–50
Nordestgaard BG (2017) A test in context: lipid profile, fasting versus nonfasting. J Am Coll Cardiol 70:1637–1646
Oscarsson J, Hurt-Camejo E (2017) Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein b-containing lipoproteins in humans: a review. Lipids Health Dis 16:149
Rajesh P, Balasubramanian K (2014) Phthalate exposure in utero causes epigenetic changes and impairs insulin signalling. J Endocrinol 223:47–66
Samblas M, Milagro FI, Martinez A (2019) DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics 14:421–444
Siddeek B, Mauduit C, Simeoni U, Benahmed M (2018) Sperm epigenome as a marker of environmental exposure and lifestyle, at the origin of diseases inheritance. Mutat Res 778:38–44
Singh S, Li SS (2012) Epigenetic effects of environmental chemicals bisphenol a and phthalates. Int J Mol Sci 13:10143–10153
Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC (2018) Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics 141:e20173459
Stevens W, Peneva D, Li JZ, Liu LZ, Liu G, Gao R, Lakdawalla DN (2016) Estimating the future burden of cardiovascular disease and the value of lipid and blood pressure control therapies in China. BMC Health Serv Res 16:175
Tamarit B, Bugault F, Pillet AH, Lavergne V, Bochet P, Garin N, Schwarz U, Thèze J, Rose T (2013) Membrane microdomains and cytoskeleton organization shape and regulate the il-7 receptor signalosome in human cd4 t-cells. J Biol Chem 288:8691–8701
Tchoukalova YD, Sarr MG, Jensen MD (2004) Measuring committed preadipocytes in human adipose tissue from severely obese patients by using adipocyte fatty acid binding protein. Am J Physiol Regul Integr Comp Physiol 287:R1132–R1140
Wharfe MD, Wyrwoll CS, Waddell BJ, Mark PJ (2016) Pregnancy suppresses the daily rhythmicity of core body temperature and adipose metabolic gene expression in the mouse. Endocrinology 157:3320–3331
Xu MX, Ge CX, Qin YT, Gu TT, Lou DS, Li Q et al (2018) Prolonged pm2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radic Biol Med 130:542–556
Yoshimura S, Nakashima S, Tomiga Y, Kawakami S, Uehara Y, Higaki Y (2018) Short- and long-term effects of high-fat diet feeding and voluntary exercise on hepatic lipid metabolism in mice. Biochem Biophys Res Commun 507:291–296
Zhang F, Ye J, Meng Y, Ai W, Su H, Zheng J, Liu F, Zhu X, Wang L, Gao P, Shu G, Jiang Q, Wang S (2018a) Calcium supplementation enhanced adipogenesis and improved glucose homeostasis through activation of camkii and pi3k/akt signaling pathway in porcine bone marrow mesenchymal stem cells (pbmscs) and mice fed high fat diet (hfd). Cell Physiol Biochem 51:154–172
Zhang H, He J, Li N, Gao N, Du Q, Chen B et al (2019a) Lipid accumulation responses in the liver of rana nigromaculata induced by perfluorooctanoic acid (pfoa). Ecotoxicol Environ Saf 167:29–35
Zhang Y, Zhang W, Fu X, Zhou F, Yu H, Na X (2018b) Transcriptomics and metabonomics analyses of maternal dehp exposure on male offspring. Environ Sci Pollut Res Int 25:26322–26329
Zhang Y, Wang S, Zhao T, Yang L, Guo S, Shi Y, Zhang X, Zhou L, Ye L (2019c) Mono-2-ethylhexyl phthalate (mehp) promoted lipid accumulation via jak2/stat5 and aggravated oxidative stress in brl-3a cells. Ecotoxicol Environ Saf 184:109611
Zhang Y, Zhou L, Zhang Z, Xu Q, Han X, Zhao Y et al (2019d) Effects of di (2-ethylhexyl) phthalate and high-fat diet on lipid metabolism in rats by jak2/stat5. Environ Sci Pollut Res Int 27:3849
Zhang YZ, Zhang ZM, Zhou LT, Zhu J, Zhang XH, Qi W, Ding S, Xu Q, Han X, Zhao YM, Song XY, Zhao TY, Ye L (2019b) Di (2-ethylhexyl) phthalate disorders lipid metabolism via tyk2/stat1 and autophagy in rats. Biomed Environ Sci: BES 32:406–418
Funding
This study was supported by the National Natural Science Foundation of China (Grant No.81972996, 81573184), and the Plan B of the Bethune Medical Department of Jilin University (2018B12).
Author information
Authors and Affiliations
Contributions
LY and LTZ designed the experiments; QX, YZZ, XH, and YMZ conducted the experiments; QX, SD, XYS, and TYZ analyzed the data; QX wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, Q., Qi, W., Zhang, Y. et al. DNA methylation of JAK3/STAT5/PPARγ regulated the changes of lipid levels induced by di (2-ethylhexyl) phthalate and high-fat diet in adolescent rats. Environ Sci Pollut Res 27, 30232–30242 (2020). https://doi.org/10.1007/s11356-020-08976-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08976-x