Abstract
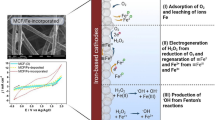
To enhance the generation of hydrogen peroxide (H2O2), a modified graphite felt cathode doped with nitrogen and boron was developed and used in peroxi-coagulation system to degrade dimethyl phthalate (DMP). After a simple modification method, the yield of H2O2 on cathode increased from 9.39 to 152.8 mg/L, with current efficiency increased from 1.61 to 70.3%. Complete degradation of DMP and 80% removal of TOC were achieved within 2 h at the optimal condition with pH of 5, cathodic potential of − 0.69 V (vs. SCE), oxygen aeration, and electrode gap of 1 cm. Possible mechanism with synergistic effect of electro-Fenton and electrocoagulation process in the peroxi-coagulation system was revealed via quenching experiments. The prospect of this system in the effluent of landfill leachate and domestic sewage was studied, achieving 50% and 61% of DMP removal in 2 h. This efficient system with simple modified cathode had promising prospects in practical applications.










Similar content being viewed by others
References
Agnoli S, Favaro M (2016) Doping graphene with boron: a review of synthesis methods, physicochemical characterization, and emerging applications. J Mater Chem A 4:5002–5025
Asghar A, Raman AAA, Daud WMAW, Ramalingam A, Zain SBM (2019) Determining the feasibility of H2O2 production at a graphite cathode using bond dissociation energy: comparing simple and nitrogen doped cathodes. Res Chem Intermed 45:3311–3327
Barrera-Diaz C, Canizares P, Fernandez FJ, Natividad R, Rodrigo MA (2014) Electrochemical advanced oxidation processes: an overview of the current applications to actual industrial effluents. J Mex Chem Soc 58:256–275
Bolton JR, Bircher KG, Tumas W, Tolman CA (2001) Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems - (IUPAC technical report). Pure Appl Chem 73:627–637
Brillas E, Garcia-Segura S (2020) Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: a review on the relevance of phenol as model molecule. Sep Purif Technol 237:116337
Brillas E, Sires I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton's reaction chemistry. Chem Rev 109:6570–6631
Casado J (2019) Towards industrial implementation of Electro-Fenton and derived technologies for wastewater treatment: a review. J Environ Chem Eng 7:102823
Chang Y, Yuan C, Li Y, Liu C, Wu T, Zeng B, Xu Y, Dai L (2017) Controllable fabrication of a N and B co-doped carbon shell on the surface of TiO2 as a support for boosting the electrochemical performances. J Mater Chem A 5:1672–1678
Chaplin BP (2014) Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ Sci-Proc Imp 16:1182–1203
Chen W, Lin S (2009) Destruction of nitrotoluenes in wastewater by electro-Fenton oxidation. J Hazard Mater 168:1562–1568
Chu Y, Zhang D, Liu L, Qian Y, Li L (2013) Electrochemical degradation of m-cresol using porous carbon-nanotube-containing cathode and Ti/SnO2-Sb2O5-IrO2 anode: kinetics, byproducts and biodegradability. J Hazard Mater 252:306–312
Colades JI, Huang CP, Retumban JD, Garcia-Segura S, de Luna MDG (2020) Electrochemically-driven dosing of iron (II) for autonomous electro-Fenton processes with in situ generation of H2O2. J Electroanal Chem 856:113639
Deng Y, Englehardt JD (2006) Treatment of landfill leachate by the Fenton process. Water Res 40:3683–3694
Ding W, Li L, Xiong K, Wang Y, Li W, Nie Y, Chen S, Qi X, Wei Z (2015) Shape fixing via salt recrystallization: a morphology-controlled approach to convert nanostructured polymer to carbon nanomaterial as a highly active catalyst for oxygen reduction reaction. J Am Chem Soc 137:5414–5420
Garcia-Segura S, Brillas E (2017) Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J Photochem Photobiol C 31:1–35
Garcia-Segura S, Salazar R, Brillas E (2013) Mineralization of phthalic acid by solar photoelectro-Fenton with a stirred boron-doped diamond/air-diffusion tank reactor: influence of Fe3+ and Cu2+ catalysts and identification of oxidation products. Electrochim Acta 113:609–619
Garcia-Segura S, Eiband MMSG, de Melo JV, Martinez-Huitle CA (2017) Electrocoagulation and advanced electrocoagulation processes: a general review about the fundamentals, emerging applications and its association with other technologies. J Electroanal Chem 801:267–299
Jiang Z, Zhao X, Tian X, Luo L, Fang J, Gao H, Jiang Z (2015) Hydrothermal synthesis of boron and nitrogen codoped hollow graphene microspheres with enhanced electrocatalytic activity for oxygen reduction reaction. ACS Appl Mater Interfaces 7:19398–19407
Kamaraj R, Vasudevan S (2019) Sulfur-doped carbon chain network as high-performance Electrocatalyst for electro-Fenton system. Chemistryselect 4:2428–2435
Kim SM, Vogelpohl A (1998) Degradation of organic pollutants by the photo-Fenton-process. Chem Eng Technol 21:187–191
Lanzarini-Lopes M, Garcia-Segura S, Hristovski K, Westerhoff P (2017) Electrical energy per order and current efficiency for electrochemical oxidation of p-chlorobenzoic acid with boron-doped diamond anode. Chemosphere 188:304–311
Lei H, Li H, Li Z, Li Z, Chen K, Zhang X, Wang H (2010) Electro-Fenton degradation of cationic red X-GRL using an activated carbon fiber cathode. Process Saf Environ 88:431–438
Li C, Huang Y, Dong X, Sun Z, Duan X, Ren B, Zheng S, Dionysiou DD (2019) Highly efficient activation of peroxymonosulfate by natural negatively-charged kaolinite with abundant hydroxyl groups for the degradation of atrazine. Appl Catal B Environ 247:10–23
Liu T, Wang K, Song S, Brouzgou A, Tsiakaras P, Wang Y (2016) New electro-Fenton gas diffusion cathode based on nitrogen-doped Graphene@carbon nanotube composite materials. Electrochim Acta 194:228–238
Liu X, Yang D, Zhou Y, Zhang J, Luo L, Meng S, Chen S, Tan M, Li Z, Tang L (2017) Electrocatalytic properties of N-doped graphite felt in electro-Fenton process and degradation mechanism of levofloxacin. Chemosphere 182:306–315
Liu Y, Feng Y, Zhang Y, Mao S, Wu D, Chu H (2019) Highly efficient degradation of dimethyl phthalate from cu(II) and dimethyl phthalate wastewater by EDTA enhanced ozonation: performance, intermediates and mechanism. J Hazard Mater 366:378–385
Molina-Garcia MA, Rees NV (2018) "metal-free" electrocatalysis: quaternary-doped graphene and the alkaline oxygen reduction reaction. Appl Catal A Gen 553:107–116
Moore NP (2000) The oestrogenic potential of the phthalate esters. Reprod Toxicol 14:183–192
Moreira FC, Boaventura RAR, Brillas E, Vilar VJP (2017) Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl Catal B Environ 202:217–261
Moreira J, Lima VB, Goulart LA, Lanza MRV (2019) Electrosynthesis of hydrogen peroxide using modified gas diffusion electrodes (MGDE) for environmental applications: quinones and azo compounds employed as redox modifiers. Appl Catal B Environ 248:95–107
Puziy AM, Poddubnaya OI, Martinez-Alonso A, Suarez-Garcia F, Tascon J (2002) Synthetic carbons activated with phosphoric acid - II. Porous structure. Carbon 40:1507–1519
Qiu S, Yu L, Tang D, Ren W, Chen K, Sun J (2018) Rapidly enhanced electro-Fenton efficiency by in situ electrochemistry-activated graphite cathode. Ind Eng Chem Res 57:4907–4915
Ren W, Peng Q, Huang Z, Zhang Z, Zhan W, Lv K, Sun J (2015) Effect of pore structure on the electro-Fenton activity of ACF@OMC cathode. Ind Eng Chem Res 54:8492–8499
Ren G, Zhou M, Su P, Liang L, Yang W, Mousset E (2018) Highly energy-efficient removal of acrylonitrile by peroxi-coagulation with modified graphite felt cathode: influence factors, possible mechanism. Chem Eng J 343:467–476
Rodrigo MA, Oturan N, Oturan MA (2014) Electrochemically assisted remediation of pesticides in soils and water: a review. Chem Rev 114:8720–8745
Sellers RM (1980) Spectrophotometric determination of hydrogen peroxide using potassium titanium(IV) oxalate. Analyst 105:950–954
Shandilya P, Mittal D, Soni M, Raizada P, Hosseini-Bandegharaei A, Saini AK, Singh P (2018) Fabrication of fluorine doped graphene and SmVO4 based dispersed and adsorptive photocatalyst for abatement of phenolic compounds from water and bacterial disinfection. J Clean Prod 203:386–399
Sheng Z, Shao L, Chen J, Bao W, Wang F, Xia X (2011) Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 5:4350–4358
Sheng Z, Gao H, Bao W, Wang F, Xia X (2012) Synthesis of boron doped graphene for oxygen reduction reaction in fuel cells. J Mater Chem 22:390–395
Wang T, Liu T (2017) Pulse electro-coagulation application in treating dibutyl phthalate wastewater. Water Sci Technol 76:1124–1131
Wang S, Iyyamperumal E, Roy A, Xue Y, Yu D, Dai L (2011) Vertically aligned BCN nanotubes as efficient metal-free electrocatalysts for the oxygen reduction reaction: a synergetic effect by co-doping with boron and nitrogen. Angew Chem Int Ed 50:11756–11760
Wang Y, Liu Y, Liu T, Song S, Gui X, Liu H, Tsiakaras P (2014) Dimethyl phthalate degradation at novel and efficient electro-Fenton cathode. Appl Catal B Environ 156:1–7
Wang D, Duan X, He X, Dionysiou DD (2016a) Degradation of dibutyl phthalate (DBP) by UV-254 nm/H2O2 photochemical oxidation: kinetics and influence of various process parameters. Environ Sci Pollut Res 23:23772–23780
Wang Y, Ao Z, Sun H, Duan X, Wang S (2016b) Activation of peroxymonosulfate by carbonaceous oxygen groups: experimental and density functional theory calculations. Appl Catal B Environ 198:295–302
Wang T, Qu G, Yin X, Sun Q, Liang D, Guo X, Jia H (2018) Dimethyl phthalate elimination from micro-polluted source water by surface discharge plasma: performance, active species roles and mechanisms. J Hazard Mater 357:279–288
Wang Y, Zhou W, Gao J, Ding Y, Kou K (2019) Oxidative modification of graphite felts for efficient H2O2 electrogeneration: enhancement mechanism and long-term stability. J Electroanal Chem 833:258–268
Wu J, Jin C, Yang Z, Tian J, Yang R (2015) Synthesis of phosphorus-doped carbon hollow spheres as efficient metal-free electrocatalysts for oxygen reduction. Carbon 82:562–571
Xu TL, Kamat PV, O'Shea KE (2005) Mechanistic evaluation of arsenite oxidation in TiO2 assisted photocatalysis. J Phys Chem A 109:9070–9075
Yao Y, Chen H, Qin J, Wu G, Lian C, Zhang J, Wang S (2016) Iron encapsulated in boron and nitrogen codoped carbon nanotubes as synergistic catalysts for Fenton-like reaction. Water Res 101:281–291
Yu F, Zhou M, Yu X (2015) Cost-effective electro-Fenton using modified graphite felt that dramatically enhanced on H2O2 electro-generation without external aeration. Electrochim Acta 163:182–189
Yu J, Liu T, Liu H, Wang Y (2016) Electro-polymerization fabrication of PANI@GF electrode and its energy-effective electrocatalytic performance in electro-Fenton process. Chin J Catal 37:2079–2085
Zhang J, Zhou W, Yang L, Chen Y, Hu Y (2018) Co-N-doped MoO2 modified carbon felt cathode for removal of EDTA-Ni in electro-Fenton process. Environ Sci Pollut Res 25:22754–22765
Zhang D, Liu T, Yin K, Liu C, Wei Y (2020) Selective H2O2 production on N-doped porous carbon from direct carbonization of metal organic frameworks for electro-Fenton mineralization of antibiotics. Chem Eng J 383:123184
Zhao L, He R, Rim KT, Schiros T, Kim KS, Zhou H, Gutierrez C, Chockalingam SP, Arguello CJ, Palova L, Nordlund D, Hybertsen MS, Reichman DR, Heinz TF, Kim P, Pinczuk A, Flynn GW, Pasupathy AN (2011) Visualizing individual nitrogen dopants in monolayer graphene. Science 333:999–1003
Zheng Y, Jiao Y, Ge L, Jaroniec M, Qiao SZ (2013) Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis. Angew Chem Int Ed 52:3110–3116
Zheng W, Xiao X, Chen B (2019) A nonradical reaction-dominated phenol degradation with peroxydisulfate catalyzed by nitrogen-doped graphene. Sci Total Environ 667:287–296
Zhou L, Hu Z, Zhang C, Bi Z, Jin T, Zhou M (2013) Electrogeneration of hydrogen peroxide for electro-Fenton system by oxygen reduction using chemically modified graphite felt cathode. Sep Purif Technol 111:131–136
Zhou L, Zhou M, Hu Z, Bi Z, Serrano KG (2014) Chemically modified graphite felt as an efficient cathode in electro-Fenton for p-nitrophenol degradation. Electrochim Acta 140:376–383
Zhou Y, Sun Y, Wang H, Zhu C, Gao J, Wu D, Huang H, Liu Y, Kang Z (2018) A nitrogen and boron co-doped metal-free carbon electrocatalyst for an efficient oxygen reduction reaction. Inorg Chem Front 5:2985–2991
Acknowledgments
The authors acknowledged the support from Open Project of State Key Laboratory of Urban Water Resource and Environment (QA201926), China Postdoctoral Science Foundation funded projects (2017M611373), Fundamental Research Funds for the Central Universities (HIT. NSRIF. 201858), and the Nature Science Foundation of Heilongjiang (E2017047).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, J., Dong, L., Geng, Y. et al. Modification of graphite felt doped with nitrogen and boron for enhanced removal of dimethyl phthalate in peroxi-coagulation system and mechanisms. Environ Sci Pollut Res 27, 18810–18821 (2020). https://doi.org/10.1007/s11356-020-08384-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08384-1