Abstract
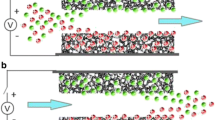
Capacitive deionization (CDI) was demonstrated to be an affordable technology for reduction of salt concentrations in brackish water. In this study, a novel membrane capacitive deionization (MCDI) cell was assembled by incorporating ion exchange membranes into the CDI cell which was built with high-adsorption electrodes based on ordered mesoporous carbon. The synthesized mesoporous carbon electrode was fully characterized. The simultaneous analysis of the electrosorption capacity and adsorption/desorption kinetics was evaluated by using real power plant desulfurization wastewater. The ordered mesoporous carbon was favorable for salt ion electrosorption, and the best performance was obtained by using MCDI which improved the removal efficiency of total dissolved solids (TDSs) from 65 to 82%. The total hardness and alkalinity of the effluent after treatment could meet the requirement of water quality standard for industries. Langmuir isotherm and pseudo-first-order kinetic models were found to be in best agreement with experimental results of salt ion electrosorption. The selective transport of ions between the electrode surface and bulk solution due to the ion exchange membranes resulted in a better desalination performance of MCDI. The results presented in this paper could be used for developing new electrode materials of MCDI for desalination from water.









Similar content being viewed by others
References
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Bayram E, Ayranci E (2010) Electrochemically enhanced removal of polycyclic aromatic basic dyes from dilute aqueous solutions by activated carbon cloth electrodes. Environ Sci Technol 44:6331–6336
Biesheuvel PM, Zhao R, Porada S, van der Wal A (2011) Theory of membrane capacitive deionization including the effect of the electrode pore space. J Colloid Interface Sci 360:239–248
El-Dessouky HT, Ettouney HM, Mandani F (2000) Performance of parallel feed multiple effect evaporation system for seawater desalination. Appl Therm Eng 20:1679–1706
Fernando WAM, Ilankoon IMSK, Syed TH, Yellishetty M (2018) Challenges and opportunities in the removal of sulphate ions in contaminated mine water: a review. Miner Eng 117:74–90
Gaikwad MS, Balomajumder C (2017) Simultaneous electrosorptive removal of chromium (VI) and fluoride ions by capacitive deionization (CDI): multicomponent isotherm modeling and kinetic study. Sep Purif Technol 186:272–281
Jacob C (2007) Seawater desalination: boron removal by ion exchange technology. Desalination 205:47–52
Jia B, Zhang W (2016) Preparation and application of electrodes in capacitive deionization (CDI): a state-of-art review. Nanoscale Res Lett 11:1–25
Karagiannis IC, Soldatos PG (2008) Water desalination cost literature: review and assessment. Desalination 223:448–456
Kim YJ, Kim JH, Choi JH (2013) Selective removal of nitrate ions by controlling the applied current in membrane capacitive deionization (MCDI). J Membr Sci 429:52–57
Kim C, Srimuk P, Lee J, Fleischmann S, Aslan M, Presser V (2017) Influence of pore structure and cell voltage of activated carbon cloth as a versatile electrode material for capacitive deionization. Carbon 122:329–335
Lee JY, Seo SJ, Yun SH, Moon SH (2011a) Preparation of ion exchanger layered electrodes for advanced membrane capacitive deionization (MCDI). Water Res 45:5375–5380
Lee KP, Arnot TC, Mattia D (2011b) A review of reverse osmosis membrane materials for desalination—development to date and future potential. J Membr Sci 370:1–22
Li Q, Sun J, Ren T, Guo L, Yang Z, Yang Q, Chen H (2017) Adsorption mechanism of 2,4-dichlorophenoxyacetic acid onto nitric acid modified activated carbon fiber. Environ Technol 39:1–10
Liang P, Yuan L, Yang X, Zhou S, Huang X (2013) Coupling ion-exchangers with inexpensive activated carbon fiber electrodes to enhance the performance of capacitive deionization cells for domestic wastewater desalination. Water Res 47:2523–2530
Liu P, Yan T, Zhang J, Shi L, Zhang D (2017) Separation and recovery of heavy metal ions and salt ions from wastewater by 3D graphene-based asymmetric electrodes via capacitive deionization. J Mater Chem A 5:14748–14757
Plazinski W, Rudzinski W, Plazinska A (2009) Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Adv. Colloid Interf Sci 152:2–13
Quan X et al (2017) Capacitive deionization of NaCl solutions with ambient pressure dried carbon aerogel microsphere electrodes. RSC Adv 7:35875–35882
Sano Y, Bai X, Amagai S, Nakayama A (2018) Effect of a porous spacer on the limiting current density in an electro-dialysis desalination. Desalination 44:141–151
Sari IP, Endarko E (2016) Enhanced salt-removal percentage in capacitive deionization with addition of ion-exchange membrane using carbon electrode synthesized with freezing thawing method. The 3rd International Conference on Advanced Materials Science & Technology (ICAMST) 1725:62–66
Seo SJ et al (2010) Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res 44:2267–2275
Show Y, Imaizumi K (2007) Electric double layer capacitor with low series resistance fabricated by carbon nanotube addition. Diam Relat Mater 16:1154–1158
Thompson J et al (2013) Rapid field assessment of RO desalination of brackish agricultural drainage water. Water Res 47:2649–2660
Wang G, Qian B, Dong Q, Yang J, Zhao Z, Qiu J (2013) Highly mesoporous activated carbon electrode for capacitive deionization. Sep Purif Technol 103:216–221
Xu Z, Xu Z (2017) Electrochemical properties of cellulose-nano-fiber/reduced graphene oxide/carbon-nano-tube aerogel. Int J Electrochem Sci 12:9335–9347
Xu S, Zhao Y, Zheng F, Zhang Y (2016) Hollow Fe3O4@mesoporous carbon core–shell microspheres for efficient sorption of radionuclides. J Mater Sci 51:2550–2557
Ying T et al (2017) Heteroatom-doped carbon materials for capacitive deionization. J Funct Mater 48:8001–8006
Zhang Y, Pinoy L, Meesschaert B, Bart VDB (2011) Separation of small organic ions from salts by ion-exchange membrane in electrodialysis. Aiche J 57:2070–2078
Zhang X, Gu P, Zhou S, Li X, Zhang G, Dong L (2018) Enhanced removal of iodide ions by nano Cu2O/Cu modified activated carbon from simulated wastewater with improved countercurrent two-stage adsorption. Sci Total Environ 626:612–620
Zhao R, Biesheuvel PM, Wal AVD (2012) Energy consumption and constant current operation in membrane capacitive deionization. Energy Environ Sci 5:9520–9527
Acknowledgements
The authors wish to thank the anonymous reviewers for their helpful suggestions to improve the paper quality.
Funding
This work is supported by the Tianjin Natural Science Foundation (Grant No. 17JCTPJC54300).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, G., Cai, W., Zhao, R. et al. Electrosorptive removal of salt ions from water by membrane capacitive deionization (MCDI): characterization, adsorption equilibrium, and kinetics. Environ Sci Pollut Res 26, 17787–17796 (2019). https://doi.org/10.1007/s11356-019-05147-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05147-5