Abstract
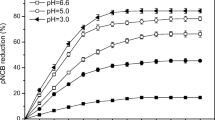
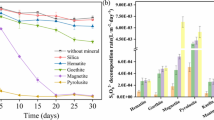
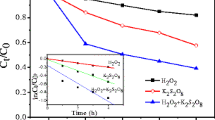
Phenylurea herbicide residuals in soil may continuously contaminate surface water and groundwater due to unregulated and improper use. Herein, we reported a stable and active oxidation system including heterogeneous Fe-based layered double hydroxide materials as persulfate (PS) activators. Under mild conditions, 1% LDH in weight and 70 mM PS can completely degrade 500 mg/kg isoproturon in soil within 10 h, during which less than 0.1 ppm heavy metal leaching was detected. This remarkable performance was consistent in a broad pH range (3~11) and was resistant to various inorganic anions (Cl−, Br−, NO3−, HCO3−) and humic acid. Mechanism studies from scavenging tests, EPR, and fluorescence spectra collectively proved that besides •OH and •SO4−, singlet oxygen (1O2) and superoxide (•O2−) were also generated and were accounted for the oxidative degradation. This unique mechanism of generating diverse radicals was clearly distinguished from classic Fe(II)/PS system, significantly reduced the influence of varying parameters in water and soil matrix, and was suggestive to chemical oxidation system in soil remediation to avoid scavenging effects by background electrolytes or other components in water/soil matrix.

ᅟ







Similar content being viewed by others
References
Ahmad M, Teel AL, Watts RJ (2010) Persulfate activation by subsurface minerals. J Contam Hydrol 115(1):34–45
Bielski BHJ, Cabelli DE, Arudi RL (1985) Reactivity of HO2/O− 2 radicals in aqueous solution. J Phys Chem Ref Data 14(4):1041–1100
Bu L, Bi C, Shi Z, Zhou S (2017) Significant enhancement on ferrous/persulfate oxidation with epigallocatechin-3-gallate: simultaneous chelating and reducing. Chem Eng J 321:642–650
Cheng X, Guo H, Zhang Y, Wu X, Liu Y (2017) Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res 113:80–88
Christoforidis KC, Louloudi M, Deligiannakis Y (2015) Effect of humic acid on chemical oxidation of organic pollutants by iron (II) and H 2 O 2: a dual mechanism. J Environ Chem Eng 3(4:2991–2996
Costa FR, Leuteritz A, Wagenknecht U, Jehnichen D, Häußler L, Heinrich G (2008) Intercalation of Mg–Al layered double hydroxide by anionic surfactants: preparation and characterization. Appl Clay Sci 38(3):153–164
Devi P, Das U, Dalai AK (2016) In-situ chemical oxidation: principle and applications of peroxide and persulfate treatments in wastewater systems. Sci Total Environ 571:643–657
Espino-Estévez MR, Fernández-Rodríguez C, González-Díaz OM, Araña J, Espinós JP, Ortega-Méndez JA, Doña-Rodríguez JM (2016) Effect of TiO2–Pd and TiO2–Ag on the photocatalytic oxidation of diclofenac, isoproturon and phenol. Chem Eng J 298:82–95
Gu X, Lu S, Li L, Qiu Z, Sui Q, Lin K, Luo Q (2011) Oxidation of 1, 1, 1-trichloroethane stimulated by thermally activated persulfate. Ind Eng Chem Res 50(19):11029–11036
Guo Y, Liu X, Han Y, Bian X, Zhang Q (2017) Effective enrichment and simultaneous quantitative analysis of trace heavy metal ions mixture in aqueous samples by the combination of radial electric focusing solid phase extraction, UV-vis spectrophotometric determination and partial least squares regression. Water Air Soil Pollut 228(8):317
Haque MM, Muneer M (2003) Heterogeneous photocatalysed degradation of a herbicide derivative, isoproturon in aqueous suspension of titanium dioxide. J Environ Manag 69(2):169–176
Hayyan M, Hashim MA, AlNashef IM (2016) Superoxide ion: generation and chemical implications. Chem Rev 116(5):3029–3085
Huang Y, Yang F, Ai LY, Feng M, Wang C, Wang Z, Liu JS (2017) On the kinetics of organic pollutant degradation with Co2+/peroxymonosulfate process: when ammonium meets chloride. Chemosphere 179:331–336
Jaafarzadeh N, Ghanbari F, Ahmadi M (2017) Efficient degradation of 2, 4-dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: a novel combination of advanced oxidation processes. Chem Eng J 320:436–447
Jawad A, Lu X, Chen Z, Yin G (2014) Degradation of chlorophenols by supported Co–Mg–Al layered double hydrotalcite with bicarbonate activated hydrogen peroxide. J Phys Chem A 118(43):10028–10035
Jawad A, Li Y, Lu X, Chen Z, Liu W, Yin G (2015) Controlled leaching with prolonged activity for Co–LDH supported catalyst during treatment of organic dyes using bicarbonate activation of hydrogen peroxide. J Hazard Mater 289:165–173
Jawad A, Liao Z, Zhou Z, Khan A, Wang T, Ifthikar J, Shahzad A, Chen Z, Chen Z (2017) Fe-MoS4: an effective and stable LDH-based adsorbent for selective removal of heavy metals. Appl Mater Interfaces 9(34):28451–28463
Jawad A, Lang J, Liao ZW, Khan A, Ifthikar J, Lv ZA, Long SJ, Chen ZL, Chen ZQ (2018) Activation of persulfate by CuOx@ Co-LDH: a novel heterogeneous system for contaminant degradation with broad pH window and controlled leaching. Chem Eng J 335:548–559
Ji Y, Ferronato C, Salvador A, Yang X, Chovelon JM (2014) Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: implications for remediation of groundwater contaminated by antibiotics. Sci Total Environ 472:800–808
Jiang X, Wu Y, Wang P, Li H, Dong W (2013) Degradation of bisphenol A in aqueous solution by persulfate activated with ferrous ion. Environ Sci Pollut Res 20:4947–4953
Johnson AC, Besien TJ, Bhardwaj CL, Dixon A, Gooddy DC, Haria AH, White C (2001) Penetration of herbicides to groundwater in an unconfined chalk aquifer following normal soil applications. J Contam Hydrol 53(1):101–117
Khan A, Liao Z, Liu Y, Jawad A, Ifthikar J, Chen Z (2017) Synergistic degradation of phenols using peroxymonosulfate activated by CuO-Co3O4@ MnO2 nanocatalyst. J Hazard Mater 329:262–271
Li S, Li M, Luo X, Huang G, Liu F, Chen H (2016) Effect of benzoic acid on the removal of 1,2-dichloroethane by a siderite-catalyzed hydrogen peroxide and persulfate system. Environ Sci Pollut Res 23:402–407
Li W, Orozco R, Camargos N, Liu H (2017a) Mechanisms on the impacts of alkalinity, pH, and chloride on persulfate-based groundwater remediation. Environ Sci Technol 51(7):3948–3959
Li X, Zhou M, Pan Y, Xu L (2017b) Pre-magnetized Fe 0/persulfate for notably enhanced degradation and dechlorination of 2, 4-dichlorophenol. Chem Eng J 307:1092–1104
Liang C, Lee IL, Hsu IY, Liang CP, Lin YL (2008) Persulfate oxidation of trichloroethylene with and without iron activation in porous media. Chemosphere 70(3):426–435
Liu H, Bruton TA, Doyle FM, Sedlak DL (2014) In situ chemical oxidation of contaminated groundwater by persulfate: decomposition by Fe (III)-and Mn (IV)-containing oxides and aquifer materials. Environ Sci Technol 48(17):10330–10336
Liu H, Bruton TA, Li W, Buren JV, Prasse C, Doyle FM, Sedlak DL (2016a) Oxidation of benzene by persulfate in the presence of Fe (III)-and Mn (IV)-containing oxides: stoichiometric efficiency and transformation products. Environ Sci Technol 50(2):890–898
Liu Y, Zhou A, Gan Y, Li X (2016b) Variability in carbon isotope fractionation of trichloroethene during degradation by persulfate activated with zero-valent iron: effects of inorganic anions. Sci Total Environ 548:1–5
Morillo E, Villaverde J (2017) Advanced technologies for the remediation of pesticide-contaminated soil. Sci Total Environ 586:576–597
Outsiou A, Frontistis Z, Ribeiro RS, Antonopoulou M, Konstantinou LK, Silva AMT, Faria JL, Gomes HT, Mantzavinos D (2017) Activation of sodium persulfate by magnetic carbon xerogels (CX/CoFe) for the oxidation of bisphenol A: process variables effects, matrix effects and reaction pathways. Water Res 124:97–107
Palmroth MRT, Langwaldt JH, Aunola TA, Goi A, Münster U, Puhakka JA, Tuhkanen TA (2006) Effect of modified Fenton’s reaction on microbial activity and removal of PAHs in creosote oil contaminated soil. Biodegradation 17(2):29–39
Pan Y, Zhou M, Li X, Xu L, Tang Z, Sheng X, Li B (2017) Highly efficient persulfate oxidation process activated with pre-magnetization Fe0. Chem Eng J 318:50–56
Parra S, Sarria V, Malato S, Péringer P, Pulgarin C (2000) Photochemical versus coupled photochemical–biological flow system for the treatment of two biorecalcitrant herbicides: metobromuron and isoproturon. Appl Catal B 27(3):153–168
Qi C, Liu X, Ma J, Lin C, Li X, Zhang H (2016) Activation of peroxymonosulfate by base: implications for the degradation of organic pollutants. Chemosphere 151:280–288
Rodriguez S, Santos A, Romero A (2017) Oxidation of priority and emerging pollutants with persulfate activated by iron: effect of iron valence and particle size. Chem Eng J 318:197–205
Sun C, Zhou R, E J, Sun J, Ren H (2015) Magnetic CuO@ Fe3O4 nanocomposite as a highly active heterogeneous catalyst of persulfate for 2, 4-dichlorophenol degradation in aqueous solution. RSC Adv 5(70):57058–57066
Tan C, Gao N, Deng Y, An N, Deng J (2012) Heat-activated persulfate oxidation of diuron in water. Chem Eng J 203:294–300
Teel AL, Ahmad M, Watts RJ (2011) Persulfate activation by naturally occurring trace minerals. J Hazard Mater 196:153–159
Venny, Gan S, Ng HK (2012) Inorganic chelated modified-Fenton treatment of polycyclic aromatic hydrocarbon (PAH)-contaminated soil. Chem Eng J 180:1–8
Waldemer RH, Tratnyek PG, Johnson RL, Nurmi JT (2007) Oxidation of chlorinated ethenes by heat-activated persulfate: kinetics and products. Environ Sci Technol 41(3):1010–1015
Wang J, Liao Z, Ifthikar J, Shi L, Du Y, Zhu J, Xi S, Chen Z, Chen Z (2017) Treatment of refractory contaminants by sludge-derived biochar/persulfate system via both adsorption and advanced oxidation process. Chemosphere 185:754–763
Xia DH, Yin R, Sun JL, An TC, Li GY, Wang WJ, Zhao H, Wong PK (2017) Natural magnetic pyrrhotite as a high-efficient persulfate activator for micropollutants degradation: radicals identification and toxicity evaluation. J Hazard Mater 340:435–444
Xiang Q, Yu J, Wong PK (2011) Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J Colloid Interface Sci 357(1):163–167
Xing M, Kong LB, Liu MC, Liu LY, Kang L, Luo YC (2014) Cobalt vanadate as highly active, stable, noble metal-free oxygen evolution electrocatalyst. J Mater Chem A 2(43):18435–18443
Xu HB, Zhao DY, Li YJ, Liu PY, Dong CX (2014) Enhanced degradation of ortho-nitrochlorobenzene by the combined system of zero-valent iron reduction and persulfate oxidation in soil. Environ Sci Pollut Res 21:5132–5140
Xu Y, Lin H, Li Y, Zhang H (2017) The mechanism and efficiency of MnO2 activated persulfate process coupled with electrolysis. Sci Total Environ 609:644–654
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci 254(8):2441–2449
Yan N, Liu F, Huang W (2013) Interaction of oxidants in siderite catalyzed hydrogen peroxide and persulfate system using trichloroethylene as a target contaminant. Chem Eng J 219:149–154
Yan J, Han L, Gao W, Xue S, Chen M (2015) Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour Technol 175:269–274
Yan S, Xiong W, Xing S, Shao Y, Guo R, Zhang H (2017) Oxidation of organic contaminant in a self-driven electro/natural maghemite/peroxydisulfate system: efficiency and mechanism. Sci Total Environ 599:1181
Zhang T, Zhu H, Croué JP (2013) Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: efficiency, stability, and mechanism. Environ Sci Technol 47(6):2784–2791
Zhao L, Hou H, Fujii A, Hosomi M, Li F (2014) Degradation of 1,4-dioxane in water with heat- and Fe2+-activated persulfate oxidation. Environ Sci Pollut Res 21:7457–7465
Acknowledgements
The authors thank the Analytical and Testing Center of Huazhong University of Science and Technology for help in XRD, FTIR, SEM, and XPS analyses.
Funding
This project was funded by the National Natural Science Foundation of China (No. 21671072), Chutian Scholar Foundation from Hubei province, and Shenzhen Science and Technology Development Funds (No. JCYJ20160429182628979).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Electronic supplementary material
ESM 1
(DOCX 1163 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Lang, J., Wang, T. et al. Enhanced degradation of isoproturon in soil through persulfate activation by Fe-based layered double hydroxide: different reactive species comparing with activation by homogenous Fe(II). Environ Sci Pollut Res 25, 26394–26404 (2018). https://doi.org/10.1007/s11356-018-2637-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2637-3