Abstract
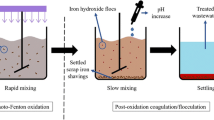
In this study, a sequential Fe0/H2O2 reaction and biological process was employed as a low-cost depth treatment method to remove recalcitrant compounds from coal-chemical engineering wastewater after regular biological treatment. First of all, a chemical oxygen demand (COD) and color removal efficiency of 66 and 63% was achieved at initial pH of 6.8, 25 mmol L−1 of H2O2, and 2 g L−1 of Fe0 in the Fe0/H2O2 reaction. According to the gas chromatography-mass spectrometer (GC-MS) and gas chromatography-flame ionization detector (GC-FID) analysis, the recalcitrant compounds were effectively decomposed into short-chain organic acids such as acetic, propionic, and butyric acids. Although these acids were resistant to the Fe0/H2O2 reaction, they were effectively eliminated in the sequential air lift reactor (ALR) at a hydraulic retention time (HRT) of 2 h, resulting in a further decrease of COD and color from 120 to 51 mg L−1 and from 70 to 38 times, respectively. A low operational cost of 0.35 $ m−3 was achieved because pH adjustment and iron-containing sludge disposal could be avoided since a total COD and color removal efficiency of 85 and 79% could be achieved at an original pH of 6.8 by the above sequential process with a ferric ion concentration below 0.8 mg L−1 after the Fe0/H2O2 reaction. It indicated that the above sequential process is a promising and cost-effective method for the depth treatment of coal-chemical engineering wastewaters to satisfy discharge requirements.






Similar content being viewed by others
References
Bergendahl JA, Thies TP (2004) Fenton’s oxidation of MTBE with zero-valent iron. Water Res 38:327–334
Bermejo MD, Cocero MJ (2006) Supercritical water oxidation: a technical review. AICHE J 52:3933–3951
Bremner DH, Burgess AE, Houllemare D, Namkung KC (2006) Phenol degradation using hydroxyl radicals generated from zero-valent iron and hydrogen peroxide. Appl Catal B Environ 63:15–19
Chen QQ, Wu PX, Dang Z, Zhu NW, Li P, Wu JH, Wang XD (2010) Iron pillared vermiculite as a heterogeneous photo-Fenton catalyst for photocatalytic degradation of azo dye reactive brilliant orange X-GN. Sep Purif Technol 71:315–323
Chen HL, Yang G, Feng YJ, Shi CL, Xu SR, Cao WP, Zhang XM (2012) Biodegradability enhancement of coking wastewater by catalytic wet air oxidation using aminated activated carbon as catalyst. Chem Eng J 198-199:45–54
Chou SS, Huang C (1999) Application of a supported iron oxyhydroxide catalyst in oxidation of benzoic acid by hydrogen peroxide. Chemosphere 38:2719–2731
Deng Y, Englehardt JD (2006) Treatment of landfill leachate by the Fenton process. Water Res 40(20):3683–3694
Du WP, Xu YM, Wang YS (2008) Photoinduced degradation of Orange II on different iron (hydr) oxides in aqueous suspension: rate enhancement on addition of hydrogen peroxide, silver nitrate, and sodium fluoride. Langmuir 24(1):175–181
Fadrus H, Malý J (1975) Suppression of iron (III) interference in the determination of iron (II) in water by the 1,10-phenanthroline method. Analyst 100:549–554
Fu FL, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205
Gallard H, Laat JD (2000) Kinetic modelling of Fe(III)/H2O2 oxidation reactions in dilute aqueous solution using atrazine as a model organic compound. Water Res 34:3107–3116
Gogate PR, Pandit AB (2004a) A review of imperative technologies for wastewater treatment I: oxidations technologies at ambient conditions. Adv Environ Res 8:501–551
Gogate PR, Pandit AB (2004b) A review of imperative technologies for wastewater treatment II: hybrid methods. Adv Environ Res 8:553–597
Hallas LE, Alexander M (1983) Microbial transformation of nitroaromatic compounds in sewage effluent. Appl Environ Microbiol 45:1234–1241
Hou BL, Han HJ, Zhuang HF, Xu P, Jia SY, Li K (2015) A novel integration of three-dimensional electro-Fenton and biological activated carbon and its application in the advanced treatment of biologically pretreated Lurgi coal gasification wastewater. Bioresour Technol 196:721–725
Huang RX, Fang ZQ, Yan XM, Cheng W (2012) Heterogeneous sono-Fenton catalytic degradation of bisphenol A by Fe3O4 magnetic nanoparticles under neutral condition. Chem Eng J 197:242–249
Jin B, Yin PH, Lant P (2006) Hydrodynamics and mass transfer coefficient in three-phase air-lift reactors containing activated sludge. Chem Eng Process 45:608–617
Kang YW, Hwang KY (2000) Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res 34:2786–2790
Lai P, Zhao HZ, Wang C, Ni JR (2007) Advanced treatment of coking wastewater by coagulation and zero-valent iron processes. J Hazard Mater 147:232–239
Lee C, Yoon J (2004) Temperature dependence of hydroxyl radical formation in the hv/Fe3+/H2O2 and Fe3+/H2O2 systems. Chemosphere 56:923–934
Liang CJ, Liang CP, Chen CC (2009) pH dependence of persulfate activation by EDTA/Fe(III) for degradation of trichloroethylene. J Contam Hydrol 106:173–182
Liu YH, Shen YJ, Yang C, Li SQ (2010) The experimental research on coking wastewater treatment by supercritical water oxidation technology. Environ Eng 28:56–59
Luo S, Qin PF, Shao JH, Peng L, Zeng QR, Gu JD (2013) Synthesis of reactive nanoscale zero valent iron using rectorite supports and its application for orange II removal. Chem Eng J 223:1–7
Martins RC, Rossi A, Quinta-Ferreira RM (2010) Fenton’s oxidation process for phenolic wastewaters remediation and biodegradability enhancement. J Hazard Mater 180:716–721
Martins RC, Lopes DV, Quina MJ, Quinta-Ferreira RM (2012) Treatment improvement of urban landfill leachates by Fenton-like process using ZVI. Chem Eng J 192:219–225
Mohammed A, Smith DW (1992) Effects of ozone on kraft process pulp mill effluent. Ozone Sci Eng 14(6):461–484
Neyens E, Baeyens J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater 98:33–50
Oncel MS, Muhcu A, Demirbas E, Kobya M (2013) A comparative study of chemical precipitation and electrocoagulation for treatment of coal acid drainage wastewater. J Environ Chem Eng 1:989–995
Rice EW, Baird RB, Eaton AD, Clesceri LS (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, Washington DC
Rubio FC, Garcia JL, Molina E, Chisti Y (2001) Axial inhomogeneities in steady-state dissolved oxygen in airlift bioreactors: predictive models. Chem Eng J 84:43–55
Ruppert G, Bauer R, Heisler G, Novalic S (1993) Mineralization of cyclic organic water contaminants by the photo-Fenton reaction: influence of structure and substituents. Chemosphere 27:1339–1347
Wang X, Jia XQ, Wen JP (2011) Transient CFD modeling of toluene waste gas biodegradation in a gas-liquid-solid three-phase airlift loop reactor by immobilized Pseudomonas putida. Chem Eng J 172:735–745
Wei XX, Zhang ZY, Fan QL, Yuan XY, Guo DS (2012) The effect of treatment stages on the coking wastewater hazardous compounds and their toxicity. J Hazard Mater 239-240:135–141
Wu HH, Dou XW, Deng DY, Guan YF, Zhang LG, He GP (2012) Decolourization of the azo dye orange G in aqueous solution via a heterogeneous Fenton-like reaction catalysed by goethite. Environ Technol 33:1545–1552
Wu JH, Chen GC, Gu JJ, Yin WZ, Lu MX, Li P, Yang B (2014) Effects of hydraulic retention time and nitrobenzene concentration on the performance of sequential upflow anaerobic filter and air lift reactors in treating nitrobenzene-containing wastewater. Environ Sci Pollut Res 21:12800–12810
Xu P, Han HJ, Bl H, Zhuang HF, Jia SY, Wang DX, Li K, Zhao Q (2015) The feasibility of using combined TiO2 photocatalysis oxidation and MBBR process for advanced treatment of biologically pretreated coal gasification wastewater. Bioresour Technol 189:417–420
Yu HQ, Gu GW, Song LP (1997) Posttreatment of effluent from coke-plant wastewater treatment system in sequencing batch reactors. J Environ Eng 123:305–308
Zhuang HF, Han HJ, Ma WC, Hou BL, Jia SY, Zhao Q (2015) Advanced treatment of biologically pretreated coal gasification wastewater by a novel heterogeneous Fenton oxidation process. J Environ Sci 33:12–20
Acknowledgements
The authors thank the support from the National Natural Science Foundation of China (41571302), the National Science and Technology Support Program Project Sub-project (2014BAC10B01-3), the Science and Technology Project of Guangdong Province (2016A010103002), the Fundamental Research Funds for the Central Universities (2017ZD025), the Water Resource Science and Technology Innovation Program of Guangdong Province (2016-26), and the State Key Laboratory of Pulp and Paper Engineering (201627).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Rights and permissions
About this article
Cite this article
Fang, Y., Yin, W., Jiang, Y. et al. Depth treatment of coal-chemical engineering wastewater by a cost-effective sequential heterogeneous Fenton and biodegradation process. Environ Sci Pollut Res 25, 13118–13126 (2018). https://doi.org/10.1007/s11356-018-1571-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1571-8